Real-World Treatment Patterns and Outcomes of Growth Hormone Treatment Among Children in Israel Over the Past Decade (2004-2015)
- PMID: 34490167
- PMCID: PMC8418062
- DOI: 10.3389/fped.2021.711979
Real-World Treatment Patterns and Outcomes of Growth Hormone Treatment Among Children in Israel Over the Past Decade (2004-2015)
Abstract
Objective: To assess a decade of growth hormone (GH) treatment patterns and outcomes in a real-world setting in Israel using a state-of-the-art computerized database. Methods: This large retrospective database study included 2,379 children initiating GH treatment in Maccabi Healthcare Services (between January 2004 and December 2014). Good adherence with therapy (proportion of days covered >80%) was assessed during follow-up. Results: At GH treatment initiation: 62.1% were boys; height standard deviation score (SDS) was -2.36 ± 0.65 (mean ± SD); age was 9.8 ± 3.1 years; and time from short stature diagnosis to first GH purchase was 4.8 ± 3.3 years. Mean treatment period was 3.5 ± 0.95 years; 79.4% of children were treated for more than 3 years. The two main indications for GH therapy were idiopathic short stature (ISS) (n = 1,615, 67.9%) and GH deficiency (GHD) (n = 611, 25.7%). Children in the highest socio-economic-status (SES) tertile comprised 61.3% of ISS and 59.7% of GHD. After 3 years, mean height gain SDS was 1.09 ± 0.91 for GHD and 0.96 ± 0.57 for ISS (p = 0.0004). Adult height (age 15 for girls and 17 for boys) was recorded for 624 patients (26.2%) with better outcomes for GHD than ISS (-1.0±0.82 vs. -1.28±0.93, respectively; p = 0.0002). Good adherence was achieved in 78.2% of the cohort during the first year and declined thereafter to 68.1% during the third year of the treatment. Conclusions: Children who initiate GH therapy are predominantly male, belong mainly to the upper SES, commence treatment a long period after initial recognition of short stature, and have suboptimal adherence. Appropriate referral, diagnosis, and follow-up care may result in better treatment outcomes with GH therapy.
Keywords: children; growth; growth hormone; growth hormone deficiency; height; idiopathic short stature.
Copyright © 2021 Ben-Ari, Chodick, Shalev, Goldstein, Gomez and Landau.
Conflict of interest statement
RG is an employee and/or stockholder of Pfizer. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from Pfizer. The funder was involved in study design and preparation of the manuscript for publication. Editorial/medical writing support was provided by Sharmy Blows, PhD, of Engage Scientific Solutions and was funded by Pfizer.
Figures

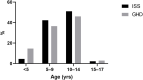

References
-
- Audi L, Fernandez-Cancio M, Camats N, Carrascosa A. Growth hormone deficiency: an update. Minerva Endocrinol. (2013) 38:1–16. - PubMed
-
- Deal C, Hasselmann C, Pfaffle RW, Zimmermann AG, Quigley CA, Child CJ, et al. Associations between pituitary imaging abnormalities and clinical and biochemical phenotypes in children with congenital growth hormone deficiency: data from an international observational study. Horm Res Paediatr. (2013) 79:283–92. 10.1159/000350829 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

