The Therapeutic Potential of Mesenchymal Stromal Cells for Regenerative Medicine: Current Knowledge and Future Understandings
- PMID: 34490235
- PMCID: PMC8416483
- DOI: 10.3389/fcell.2021.661532
The Therapeutic Potential of Mesenchymal Stromal Cells for Regenerative Medicine: Current Knowledge and Future Understandings
Abstract
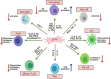
In recent decades, research on the therapeutic potential of progenitor cells has advanced considerably. Among progenitor cells, mesenchymal stromal cells (MSCs) have attracted significant interest and have proven to be a promising tool for regenerative medicine. MSCs are isolated from various anatomical sites, including bone marrow, adipose tissue, and umbilical cord. Advances in separation, culture, and expansion techniques for MSCs have enabled their large-scale therapeutic application. This progress accompanied by the rapid improvement of transplantation practices has enhanced the utilization of MSCs in regenerative medicine. During tissue healing, MSCs may exhibit several therapeutic functions to support the repair and regeneration of injured tissue. The process underlying these effects likely involves the migration and homing of MSCs, as well as their immunotropic functions. The direct differentiation of MSCs as a cell replacement therapeutic mechanism is discussed. The fate and behavior of MSCs are further regulated by their microenvironment, which may consequently influence their repair potential. A paracrine pathway based on the release of different messengers, including regulatory factors, chemokines, cytokines, growth factors, and nucleic acids that can be secreted or packaged into extracellular vesicles, is also implicated in the therapeutic properties of MSCs. In this review, we will discuss relevant outcomes regarding the properties and roles of MSCs during tissue repair and regeneration. We will critically examine the influence of the local microenvironment, especially immunological and inflammatory signals, as well as the mechanisms underlying these therapeutic effects. Importantly, we will describe the interactions of local progenitor and immune cells with MSCs and their modulation during tissue injury. We will also highlight the crucial role of paracrine pathways, including the role of extracellular vesicles, in this healing process. Moreover, we will discuss the therapeutic potential of MSCs and MSC-derived extracellular vesicles in the treatment of COVID-19 (coronavirus disease 2019) patients. Overall, this review will provide a better understanding of MSC-based therapies as a novel immunoregenerative strategy.
Keywords: cell therapy; immunomodulation; mesenchymal stromal cells; paracrine mechanisms; regenerative medicine; trophic function.
Copyright © 2021 Merimi, El-Majzoub, Lagneaux, Moussa Agha, Bouhtit, Meuleman, Fahmi, Lewalle, Fayyad-Kazan and Najar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

