G-Protein-Coupled Estrogen Receptor-1 Positively Regulates the Growth Plate Chondrocyte Proliferation in Female Pubertal Mice
- PMID: 34490260
- PMCID: PMC8417792
- DOI: 10.3389/fcell.2021.710664
G-Protein-Coupled Estrogen Receptor-1 Positively Regulates the Growth Plate Chondrocyte Proliferation in Female Pubertal Mice
Abstract
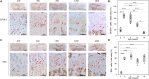
Estrogen enhances long bone longitudinal growth during early puberty. Growth plate chondrocytes are the main cells that contribute to long bone elongation. The role of G-protein-coupled estrogen receptor-1 (GPER-1) in regulating growth plate chondrocyte function remains unclear. In the present study, we generated chondrocyte-specific GPER-1 knockout (CKO) mice to investigate the effect of GPER-1 in growth plate chondrocytes. In control mice, GPER-1 was highly expressed in the growth plates of 4- and 8-week-old mice, with a gradual decline through 12 to 16 weeks. In CKO mice, the GPER-1 expression in growth plate chondrocytes was significantly lower than that in the control mice (80% decrease). The CKO mice also showed a decrease in body length (crown-rump length), body weight, and the length of tibias and femurs at 8 weeks. More importantly, the cell number and thickness of the proliferative zone of the growth plate, as well as the thickness of primary spongiosa and length of metaphysis plus diaphysis in tibias of CKO mice, were significantly decreased compared with those of the control mice. Furthermore, there was also a considerable reduction in the number of proliferating cell nuclear antigens and Ki67-stained proliferating chondrocytes in the tibia growth plate in the CKO mice. The chondrocyte proliferation mediated by GPER-1 was further demonstrated via treatment with a GPER-1 antagonist in cultured epiphyseal cartilage. This study demonstrates that GPER-1 positively regulates chondrocyte proliferation at the growth plate during early puberty and contributes to the longitudinal growth of long bones.
Keywords: G-protein-coupled estrogen receptor-1; bone growth; chondrocyte-specific knockout mice; estrogen receptor; long bone elongation.
Copyright © 2021 Chou, Chuang, Chen, Ho and Chang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Chagin A. S., Savendahl L. (2007a). Estrogens and growth: review. Pediatr. Endocrinol. Rev. 4 329–334. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

