Estimation and Discriminability of Doppler Ultrasound Fetal Heart Rate Variability Measures
- PMID: 34490419
- PMCID: PMC8417534
- DOI: 10.3389/frai.2021.674238
Estimation and Discriminability of Doppler Ultrasound Fetal Heart Rate Variability Measures
Abstract
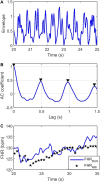
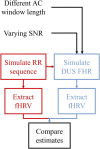
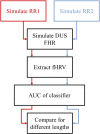
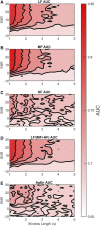
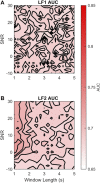
Continuous electronic fetal monitoring and the access to databases of fetal heart rate (FHR) data have sparked the application of machine learning classifiers to identify fetal pathologies. However, most fetal heart rate data are acquired using Doppler ultrasound (DUS). DUS signals use autocorrelation (AC) to estimate the average heartbeat period within a window. In consequence, DUS FHR signals loses high frequency information to an extent that depends on the length of the AC window. We examined the effect of this on the estimation bias and discriminability of frequency domain features: low frequency power (LF: 0.03-0.15 Hz), movement frequency power (MF: 0.15-0.5 Hz), high frequency power (HF: 0.5-1 Hz), the LF/(MF + HF) ratio, and the nonlinear approximate entropy (ApEn) as a function of AC window length and signal to noise ratio. We found that the average discriminability loss across all evaluated AC window lengths and SNRs was 10.99% for LF 14.23% for MF, 13.33% for the HF, 10.39% for the LF/(MF + HF) ratio, and 24.17% for ApEn. This indicates that the frequency domain features are more robust to the AC method and additive noise than the ApEn. This is likely because additive noise increases the irregularity of the signals, which results in an overestimation of ApEn. In conclusion, our study found that the LF features are the most robust to the effects of the AC method and noise. Future studies should investigate the effect of other variables such as signal drop, gestational age, and the length of the analysis window on the estimation of fHRV features and their discriminability.
Keywords: Doppler ultrasound; autocorrelation; cardiotocography; classification; fetal heart rate; fetal heart rate variability.
Copyright © 2021 Vargas-Calixto, Warrick and Kearney.
Conflict of interest statement
Author PW was employed by the company PeriGen Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
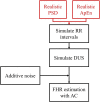


References
-
- American College of Obstetricians and Gynecologists (2014). “Intrapartum Considerations and Assessment,” in Neonatal Encephalopathy and Neurologic Outcome, Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy (Washington, DC: American Academy of Pediatrics; ), 87–114.
-
- Campanile M., D’Alessandro P., Della Corte L., Saccone G., Tagliaferri S., Arduino B., et al. (2018). Intrapartum Cardiotocography with and without Computer Analysis: a Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Maternal-Fetal Neonatal Med. 33, 2284–2290. 10.1080/14767058.2018.1542676 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

