Ductular reaction promotes intrahepatic angiogenesis through Slit2-Roundabout 1 signaling
- PMID: 34490644
- PMCID: PMC8766889
- DOI: 10.1002/hep.32140
Ductular reaction promotes intrahepatic angiogenesis through Slit2-Roundabout 1 signaling
Abstract
Background and aims: Ductular reaction (DR) expands in chronic liver diseases and correlates with disease severity. Besides its potential role in liver regeneration, DR plays a role in the wound-healing response of the liver, promoting periductular fibrosis and inflammatory cell recruitment. However, there is no information regarding its role in intrahepatic angiogenesis. In the current study we investigated the potential contribution of DR cells to hepatic vascular remodeling during chronic liver disease.
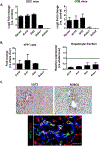
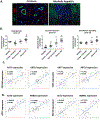
Approach and results: In mouse models of liver injury, DR cells express genes involved in angiogenesis. Among angiogenesis-related genes, the expression of Slit2 and its receptor Roundabout 1 (Robo1) was localized in DR cells and neoangiogenic vessels, respectively. The angiogenic role of the Slit2-Robo1 pathway in chronic liver disease was confirmed in ROBO1/2-/+ mice treated with 3,5-diethoxycarbonyl-1,4-dihydrocollidine, which displayed reduced intrahepatic neovascular density compared to wild-type mice. However, ROBO1/2 deficiency did not affect angiogenesis in partial hepatectomy. In patients with advanced alcohol-associated disease, angiogenesis was associated with DR, and up-regulation of SLIT2-ROBO1 correlated with DR and disease severity. In vitro, human liver-derived organoids produced SLIT2 and induced tube formation of endothelial cells.
Conclusions: Overall, our data indicate that DR expansion promotes angiogenesis through the Slit2-Robo1 pathway and recognize DR cells as key players in the liver wound-healing response.
© 2021 The Authors. Hepatology published by Wiley Periodicals LLC on behalf of American Association for the Study of Liver Diseases.
Conflict of interest statement
Figures




References
-
- Deng X, Zhang X, Li W, Feng RX, Li L, Yi GR, et al. Chronic Liver Injury Induces Conversion of Biliary Epithelial Cells into Hepatocytes. Cell Stem Cell. 2018. Jul 5;23(1):114–122.e3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

