Host-pathogen interactions of clinical S. aureus isolates to induce infective endocarditis
- PMID: 34490828
- PMCID: PMC8425731
- DOI: 10.1080/21505594.2021.1960107
Host-pathogen interactions of clinical S. aureus isolates to induce infective endocarditis
Abstract
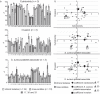
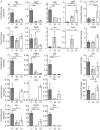
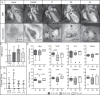
To evaluate potential pathomechanisms in the induction of infective endocarditis (IE), 34 Staphylococcus aureus (S. aureus) isolates, collected from patients with S. aureus endocarditis and from healthy individuals were investigated both in vitro and in vivo. S. aureus isolates were tested in vitro for their cytotoxicity, invasion and the association with platelets. Virulence factor expression profiles and cellular response were additionally investigated and tested for correlation with the ability of S. aureus to induce vegetations on the aortic valves in vivo. In an animal model of IE valvular conspicuity was assessed by in vivo magnetic resonance imaging at 9.4 T, histology and enrichment gene expression analysis. All S. aureus isolates tested in vivo caused a reliable infection and inflammation of the aortic valves, but could not be differentiated and categorized according to the measured in vitro virulence profiles and cytotoxicity. Results from in vitro assays did not correlate with the severity of IE. However, the isolates differed substantially in the activation and inhibition of pathways connected to the extracellular matrix and inflammatory response. Thus, comprehensive approaches of host-pathogen interactions and corresponding immune pathways are needed for the evaluation of the pathogenic capacity of bacteria. An improved understanding of the interaction between virulence factors and immune response in S. aureus infective endocarditis would offer novel possibilities for the development of therapeutic strategies and specific diagnostic imaging markers.
Keywords: MRI; Staphylococcus aureus; clinical isolates; endocarditis; pathomechanisms.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


Similar articles
-
S. aureus endocarditis: Clinical aspects and experimental approaches.Int J Med Microbiol. 2018 Aug;308(6):640-652. doi: 10.1016/j.ijmm.2018.02.004. Epub 2018 Feb 21. Int J Med Microbiol. 2018. PMID: 29526448 Review.
-
The microbiological characteristics of Staphylococcus aureus isolated from patients with native valve infective endocarditis.Virulence. 2019 Dec;10(1):948-956. doi: 10.1080/21505594.2019.1685631. Virulence. 2019. PMID: 31718473 Free PMC article.
-
Staphylococcus aureus CC30 Lineage and Absence of sed,j,r-Harboring Plasmid Predict Embolism in Infective Endocarditis.Front Cell Infect Microbiol. 2018 Jun 8;8:187. doi: 10.3389/fcimb.2018.00187. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29938201 Free PMC article.
-
Molecular characterization of endocarditis-associated Staphylococcus aureus.J Clin Microbiol. 2013 Jul;51(7):2131-8. doi: 10.1128/JCM.00651-13. Epub 2013 Apr 24. J Clin Microbiol. 2013. PMID: 23616460 Free PMC article.
-
Inflammasome Activation Can Mediate Tissue-Specific Pathogenesis or Protection in Staphylococcus aureus Infection.Curr Top Microbiol Immunol. 2016;397:257-82. doi: 10.1007/978-3-319-41171-2_13. Curr Top Microbiol Immunol. 2016. PMID: 27460814 Free PMC article. Review.
Cited by
-
Infective Endocarditis in High-Income Countries.Metabolites. 2022 Jul 25;12(8):682. doi: 10.3390/metabo12080682. Metabolites. 2022. PMID: 35893249 Free PMC article. Review.
-
Neutrophils Protect Against Staphylococcus aureus Endocarditis Progression Independent of Extracellular Trap Release.Arterioscler Thromb Vasc Biol. 2023 Feb;43(2):267-285. doi: 10.1161/ATVBAHA.122.317800. Epub 2022 Dec 1. Arterioscler Thromb Vasc Biol. 2023. PMID: 36453281 Free PMC article.
-
Staphylococcus aureus Endocarditis Immunothrombosis.Metabolites. 2025 May 15;15(5):328. doi: 10.3390/metabo15050328. Metabolites. 2025. PMID: 40422904 Free PMC article. Review.
-
Staphylococcus aureus Infections and Human Intestinal Microbiota.Pathogens. 2024 Mar 24;13(4):276. doi: 10.3390/pathogens13040276. Pathogens. 2024. PMID: 38668232 Free PMC article. Review.
-
Commentary: "Käytännöllistelemälläkin": Antiaggregants, anticoagulants, and endocarditis.JTCVS Open. 2021 Nov 6;8:313-314. doi: 10.1016/j.xjon.2021.11.004. eCollection 2021 Dec. JTCVS Open. 2021. PMID: 36004159 Free PMC article. No abstract available.
References
-
- Hoen B, Duval X.. Clinical practice. Infective endocarditis. N Engl J Med. 2013;368(15):1425–1433. - PubMed
-
- Werdan K, Dietz S, Loffler B, et al. Mechanisms of infective endocarditis: pathogen–host interaction and risk states. Nat Rev Cardiol. 2014;11(1):35–50. - PubMed
-
- Niemann S, Spehr N, van Aken H, et al. Soluble fibrin is the main mediator of staphylococcus aureus adhesion to platelets. Circulation. 2004;110(2):193–200. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
