Arcuate Nucleus-Dependent Regulation of Metabolism-Pathways to Obesity and Diabetes Mellitus
- PMID: 34490882
- PMCID: PMC8905335
- DOI: 10.1210/endrev/bnab025
Arcuate Nucleus-Dependent Regulation of Metabolism-Pathways to Obesity and Diabetes Mellitus
Abstract
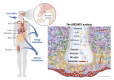
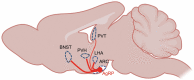
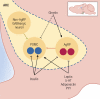
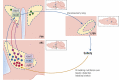
The central nervous system (CNS) receives information from afferent neurons, circulating hormones, and absorbed nutrients and integrates this information to orchestrate the actions of the neuroendocrine and autonomic nervous systems in maintaining systemic metabolic homeostasis. Particularly the arcuate nucleus of the hypothalamus (ARC) is of pivotal importance for primary sensing of adiposity signals, such as leptin and insulin, and circulating nutrients, such as glucose. Importantly, energy state-sensing neurons in the ARC not only regulate feeding but at the same time control multiple physiological functions, such as glucose homeostasis, blood pressure, and innate immune responses. These findings have defined them as master regulators, which adapt integrative physiology to the energy state of the organism. The disruption of this fine-tuned control leads to an imbalance between energy intake and expenditure as well as deregulation of peripheral metabolism. Improving our understanding of the cellular, molecular, and functional basis of this regulatory principle in the CNS could set the stage for developing novel therapeutic strategies for the treatment of obesity and metabolic syndrome. In this review, we summarize novel insights with a particular emphasis on ARC neurocircuitries regulating food intake and glucose homeostasis and sensing factors that inform the brain of the organismal energy status.
Keywords: arcuate nucleus; energy homeostasis; feeding; hypothalamus; obesity; type 2 diabetes mellitus.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society.
Figures

References
-
- Bluher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288-298. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

