Validation of the Surrogate Threshold Effect for Change in Bone Mineral Density as a Surrogate Endpoint for Fracture Outcomes: The FNIH-ASBMR SABRE Project
- PMID: 34490915
- PMCID: PMC9291617
- DOI: 10.1002/jbmr.4433
Validation of the Surrogate Threshold Effect for Change in Bone Mineral Density as a Surrogate Endpoint for Fracture Outcomes: The FNIH-ASBMR SABRE Project
Abstract
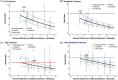
The surrogate threshold effect (STE) is defined as the minimum treatment effect on a surrogate that is reliably predictive of a treatment effect on the clinical outcome. It provides a framework for implementing a clinical trial with a surrogate endpoint. The aim of this study was to update our previous analysis by validating the STE for change in total hip (TH) BMD as a surrogate for fracture risk reduction; the novelty of this study was this validation. To do so, we used individual patient data from 61,415 participants in 16 RCTs that evaluated bisphosphonates (nine trials), selective estrogen receptor modulators (four trials), denosumab (one trial), odanacatib (one trial), and teriparatide (one trial) to estimate trial-specific treatment effects on TH BMD and all, vertebral, hip, and nonvertebral fractures. We then conducted a random effects meta-regression of the log relative fracture risk reduction against 24-month change in TH BMD, and computed the STE as the intersection of the upper 95% prediction limit of this regression with the line of no fracture reduction. We validated the STE by checking whether the number of fractures in each trial provided 80% power and determining what proportion of trials with BMD changes ≥ STE reported significant reductions in fracture risk. We applied this analysis to (i) the trials on which we estimated the STE; and (ii) trials on which we did not estimate the STE. We found that the STEs for all, vertebral, hip, and nonvertebral fractures were 1.83%, 1.42%, 3.18%, and 2.13%, respectively. Among trials used to estimate STE, 27 of 28 were adequately powered, showed BMD effects exceeding the STE, and showed significant reductions in fracture risk. Among the validation set of 11 trials, 10 met these criteria. Thus STE differs by fracture type and has been validated in trials not used to develop the approach. © 2021 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Keywords: BISPHOSPHONATES; BONE MINERAL DENSITY; FRACTURE; SELECTIVE ESTROGEN RECEPTOR MODULATORS; SURROGATE.
© 2021 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Figures
Similar articles
-
Diabetes Mellitus and the Benefit of Antiresorptive Therapy on Fracture Risk.J Bone Miner Res. 2022 Nov;37(11):2121-2131. doi: 10.1002/jbmr.4697. Epub 2022 Oct 7. J Bone Miner Res. 2022. PMID: 36065588 Free PMC article. Clinical Trial.
-
The relationship between baseline bone mineral density and fracture incidence in the placebo groups of randomized controlled trials using individual patient data from the FNIH-ASBMR-SABRE project.J Bone Miner Res. 2025 Mar 15;40(3):307-314. doi: 10.1093/jbmr/zjae201. J Bone Miner Res. 2025. PMID: 39680674 Free PMC article.
-
Pre-treatment bone mineral density and the benefit of pharmacologic treatment on fracture risk and BMD change: analysis from the FNIH-ASBMR SABRE project.J Bone Miner Res. 2024 Aug 5;39(7):867-876. doi: 10.1093/jbmr/zjae068. J Bone Miner Res. 2024. PMID: 38691441 Free PMC article.
-
Treatment-related changes in bone mineral density as a surrogate biomarker for fracture risk reduction: meta-regression analyses of individual patient data from multiple randomised controlled trials.Lancet Diabetes Endocrinol. 2020 Aug;8(8):672-682. doi: 10.1016/S2213-8587(20)30159-5. Lancet Diabetes Endocrinol. 2020. PMID: 32707115
-
Denosumab, raloxifene, romosozumab and teriparatide to prevent osteoporotic fragility fractures: a systematic review and economic evaluation.Health Technol Assess. 2020 Jun;24(29):1-314. doi: 10.3310/hta24290. Health Technol Assess. 2020. PMID: 32588816 Free PMC article.
Cited by
-
Efficacy of osteoporosis pharmacological treatments in men: a systematic review and meta-analysis.Aging Clin Exp Res. 2023 Sep;35(9):1789-1806. doi: 10.1007/s40520-023-02478-9. Epub 2023 Jul 3. Aging Clin Exp Res. 2023. PMID: 37400668 Free PMC article.
-
Osteoporosis in Older Men: Informing Patient Management and Improving Health-Related Outcomes.Drugs Aging. 2025 Jan;42(1):21-38. doi: 10.1007/s40266-024-01163-4. Epub 2025 Jan 8. Drugs Aging. 2025. PMID: 39775765 Review.
-
The effectiveness of ibandronate in reducing the risk of nonvertebral fractures in women with osteoporosis: systematic review and meta-analysis of observational studies.Int J Clin Pharm. 2024 Apr;46(2):357-367. doi: 10.1007/s11096-023-01666-x. Epub 2023 Dec 19. Int J Clin Pharm. 2024. PMID: 38112890 Free PMC article.
-
The Efficacy and Safety of Abaloparatide-SC in Men With Osteoporosis: A Randomized Clinical Trial.J Bone Miner Res. 2022 Dec;37(12):2435-2442. doi: 10.1002/jbmr.4719. Epub 2022 Oct 18. J Bone Miner Res. 2022. PMID: 36190391 Free PMC article. Clinical Trial.
-
Intravenous Zoledronate 4 mg for the treatment of post-menopausal osteoporosis: A prospective open-labeled study.Bone Rep. 2021 Nov 26;16:101153. doi: 10.1016/j.bonr.2021.101153. eCollection 2022 Jun. Bone Rep. 2021. PMID: 34926731 Free PMC article.
References
-
- Black DM, Bauer DC, Vittinghoff E, et al. Treatment‐related changes in bone mineral density as a surrogate biomarker for fracture risk reduction: meta‐regression analyses of individual patient data from multiple randomised controlled trials. Lancet Diabetes Endocrinol. 2020;8(8):672‐682. - PubMed
-
- Miller PD. Bone strength and surrogate markers: the first, second, and third fiddle. J Bone Miner Res. 2012;27(8):1623‐1626. - PubMed
-
- Ciani O, Buyse M, Drummond M, Rasi G, Saad ED, Taylor RS. Time to review the role of surrogate end points in health policy: state of the art and the way forward. Value Health. 2017;20(3):487‐495. - PubMed
-
- Amur S, LaVange L, Zineh I, Buckman‐Garner S, Woodcock J. Biomarker qualification: toward a multiple stakeholder framework for biomarker development, regulatory acceptance, and utilization. Clin Pharmacol Ther. 2015;98(1):34‐46. - PubMed
-
- Johnson KR, Freemantle N, Anthony DM, Lassere MN. LDL‐cholesterol differences predicted survival benefit in statin trials by the surrogate threshold effect (STE). J Clin Epidemiol. 2009;62(3):328‐336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

