Cyclic Tetrapeptide HDAC Inhibitors with Improved Plasmodium falciparum Selectivity and Killing Profile
- PMID: 34491031
- PMCID: PMC8822587
- DOI: 10.1021/acsinfecdis.1c00341
Cyclic Tetrapeptide HDAC Inhibitors with Improved Plasmodium falciparum Selectivity and Killing Profile
Abstract
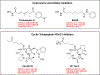
Cyclic tetrapeptide histone deacetylase inhibitors represent a promising class of antiplasmodial agents that epigenetically disrupt a wide range of cellular processes in Plasmodium falciparum. Unfortunately, certain limitations, including reversible killing effects and host cell toxicity, prevented these inhibitors from further development and clinical use as antimalarials. In this study, we present a series of cyclic tetrapeptide analogues derived primarily from the fungus Wardomyces dimerus that inhibit P. falciparum with low nanomolar potency and high selectivity. This cyclic tetrapeptide scaffold was diversified further via semisynthesis, leading to the identification of several key structural changes that positively impacted the selectivity, potency, and in vitro killing profiles of these compounds. We confirmed their effectiveness as HDAC inhibitors through the inhibition of PfHDAC1 catalytic activity, in silico modeling, and the hyperacetylation of histone H4. Additional analysis revealed the in vitro inhibition of the most active epoxide-containing analogue was plasmodistatic, exhibiting reversible inhibitory effects upon compound withdrawal after 24 or 48 h. In contrast, one of the new diacetyloxy semisynthetic analogues, CTP-NPDG 19, displayed a rapid and irreversible action against the parasite following compound exposure for 24 h.
Keywords: HDAC; PfHDAC1; Plasmodium; cyclic tetrapeptide; malaria; natural products.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

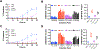
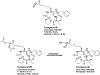


References
-
- World Health Organization, G. M. P. World malaria report 2020; 2020.
-
- WHO Artemisinin resistance and artemisinin-based combination therapy efficacy World Health Organization: 2019.
-
- Straimer J; Gnadig NF; Witkowski B; Amaratunga C; Duru V; Ramadani AP; Dacheux M; Khim N; Zhang L; Lam S; Gregory PD; Urnov FD; Mercereau-Puijalon O; Benoit-Vical F; Fairhurst RM; Menard D; Fidock DA, Drug resistance. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science 2015, 347 (6220), 428–431. DOI: 10.1126/science.1260867. - DOI - PMC - PubMed
-
- Mathieu LC; Cox H; Early AM; Mok S; Lazrek Y; Paquet JC; Ade MP; Lucchi NW; Grant Q; Udhayakumar V; Alexandre JS; Demar M; Ringwald P; Neafsey DE; Fidock DA; Musset L, Local emergence in Amazonia of Plasmodium falciparum k13 C580Y mutants associated with in vitro artemisinin resistance. Elife 2020, 9, 1–21. DOI: 10.7554/eLife.51015. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

