Glutamate Dehydrogenase (GdhA) of Streptococcus pneumoniae Is Required for High Temperature Adaptation
- PMID: 34491792
- PMCID: PMC8594611
- DOI: 10.1128/IAI.00400-21
Glutamate Dehydrogenase (GdhA) of Streptococcus pneumoniae Is Required for High Temperature Adaptation
Abstract
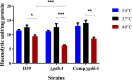
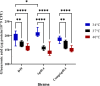
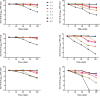
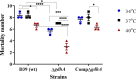
During its progression from the nasopharynx to other sterile and nonsterile niches of its human host, Streptococcus pneumoniae must cope with changes in temperature. We hypothesized that the temperature adaptation is an important facet of pneumococcal survival in the host. Here, we evaluated the effect of temperature on pneumococcus and studied the role of glutamate dehydrogenase (GdhA) in thermal adaptation associated with virulence and survival. Microarray analysis revealed a significant transcriptional response to changes in temperature, affecting the expression of 252 genes in total at 34°C and 40°C relative to at 37°C. One of the differentially regulated genes was gdhA, which is upregulated at 40°C and downregulated at 34°C relative to 37°C. Deletion of gdhA attenuated the growth, cell size, biofilm formation, pH survival, and biosynthesis of proteins associated with virulence in a temperature-dependent manner. Moreover, deletion of gdhA stimulated formate production irrespective of temperature fluctuation. Finally, ΔgdhA grown at 40°C was less virulent than other temperatures or the wild type at the same temperature in a Galleria mellonella infection model, suggesting that GdhA is required for pneumococcal virulence at elevated temperature.
Keywords: CcpA; Galleria mellonella; GdhA; Streptococcus pneumoniae; temperature; transcriptional expression.
Figures



References
-
- Eichner H, Pathak A, Henriques-Normark B, Loh E. 2019. Meningitis pathogens evade immune responses by thermosensing. bioRxiv 10.1101/586131. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

