Safety, Tolerability, Pharmacokinetics, and Immunogenicity of a Monoclonal Antibody (SCTA01) Targeting SARS-CoV-2 in Healthy Adults: a Randomized, Double-Blind, Placebo-Controlled, Phase I Study
- PMID: 34491805
- PMCID: PMC8522782
- DOI: 10.1128/AAC.01063-21
Safety, Tolerability, Pharmacokinetics, and Immunogenicity of a Monoclonal Antibody (SCTA01) Targeting SARS-CoV-2 in Healthy Adults: a Randomized, Double-Blind, Placebo-Controlled, Phase I Study
Abstract
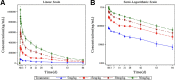
SCTA01 is a novel monoclonal antibody with promising prophylactic and therapeutic potential for COVID-19. This study aimed to evaluate the safety, tolerability, pharmacokinetics (PK) and immunogenicity of SCTA01 in healthy adults. This was a randomized, double-blind, placebo-controlled, dose escalation phase I clinical trial. Healthy adults were randomly assigned to cohort 1 (n = 5; 3:2), cohort 2 (n = 8; 6:2), cohort 3, or cohort 4 (both n = 10; 8:2) to receive SCTA01 (5, 15, 30, and 50 mg/kg, respectively) versus placebo. All participants were followed up for clinical, laboratory, PK, and immunogenicity assessments for 84 days. The primary outcomes were the dose-limiting toxicity (DLT) and maximal tolerable dose (MTD), and the secondary outcomes included PK parameters, immunogenicity, and adverse events (AE). Of the 33 participants, 18 experienced treatment-related AEs; the frequency was 52.0% (13/25) in participants receiving SCTA01 and 62.5% (5/8) in those receiving placebo. All AEs were mild. There was no serious AE or death. No DLT was reported, and the MTD of SCTA01 was not reached. SCTA01 with a dose range of 5 to 50 mg/kg had nearly linear dose-proportional increases in Cmax and AUC parameters. An antidrug antibody response was detected in four (16.0%) participants receiving SCTA01, with low titers, between the baseline and day 28, but all became negative later. In conclusion, SCTA01 up to 50 mg/kg was safe and well-tolerated in healthy participants. Its PK parameters were nearly linear dose-proportional. (This study has been registered at ClinicalTrials.gov under identifier NCT04483375.).
Keywords: COVID-19; SARS-CoV-2; monoclonal antibody; pharmacokinetics; safety.
Figures
Similar articles
-
Safety, tolerability, pharmacokinetic characteristics, and immunogenicity of MW33: a Phase 1 clinical study of the SARS-CoV-2 RBD-targeting monoclonal antibody.Emerg Microbes Infect. 2021 Dec;10(1):1638-1648. doi: 10.1080/22221751.2021.1960900. Emerg Microbes Infect. 2021. PMID: 34346827 Free PMC article. Clinical Trial.
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Safety, Virologic Efficacy, and Pharmacokinetics of CT-P59, a Neutralizing Monoclonal Antibody Against SARS-CoV-2 Spike Receptor-Binding Protein: Two Randomized, Placebo-Controlled, Phase I Studies in Healthy Individuals and Patients With Mild SARS-CoV-2 Infection.Clin Ther. 2021 Oct;43(10):1706-1727. doi: 10.1016/j.clinthera.2021.08.009. Epub 2021 Aug 23. Clin Ther. 2021. PMID: 34551869 Free PMC article. Clinical Trial.
-
Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial.Lancet Infect Dis. 2021 Jun;21(6):803-812. doi: 10.1016/S1473-3099(20)30987-7. Epub 2021 Feb 3. Lancet Infect Dis. 2021. PMID: 33548194 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of an inactivated whole virion SARS-CoV-2 vaccine, TURKOVAC, in healthy adults: Interim results from randomised, double-blind, placebo-controlled phase 1 and 2 trials.Vaccine. 2023 Jan 9;41(2):380-390. doi: 10.1016/j.vaccine.2022.10.093. Epub 2022 Nov 22. Vaccine. 2023. PMID: 36460536 Free PMC article.
Cited by
-
Advances in Pathogenesis, Progression, Potential Targets and Targeted Therapeutic Strategies in SARS-CoV-2-Induced COVID-19.Front Immunol. 2022 Apr 5;13:834942. doi: 10.3389/fimmu.2022.834942. eCollection 2022. Front Immunol. 2022. PMID: 35450063 Free PMC article. Review.
-
Casirivimab-imdevimab neutralizing SARS-CoV-2: post-infusion clinical events and their risk factors.J Pharm Health Care Sci. 2022 Jan 3;8(1):1. doi: 10.1186/s40780-021-00233-8. J Pharm Health Care Sci. 2022. PMID: 34980269 Free PMC article.
-
Antibody-mediated neutralization of SARS-CoV-2.Immunity. 2022 Jun 14;55(6):925-944. doi: 10.1016/j.immuni.2022.05.005. Epub 2022 May 13. Immunity. 2022. PMID: 35623355 Free PMC article. Review.
-
Fc-Modified Antibody in Hospitalized Severe COVID-19 Patients.Vaccines (Basel). 2025 Mar 31;13(4):372. doi: 10.3390/vaccines13040372. Vaccines (Basel). 2025. PMID: 40333222 Free PMC article.
-
Efficacy and safety of SARS-CoV-2 neutralizing antibody, SCTA01, in high-risk outpatients diagnosed with COVID-19: A Phase II clinical trial.Contemp Clin Trials Commun. 2025 May 17;45:101496. doi: 10.1016/j.conctc.2025.101496. eCollection 2025 Jun. Contemp Clin Trials Commun. 2025. PMID: 40520909 Free PMC article.
References
-
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. 2020. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 382:1199–1207. 10.1056/NEJMoa2001316. - DOI - PMC - PubMed
-
- Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. 2020. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 395:1569–1578. 10.1016/S0140-6736(20)31022-9. - DOI - PMC - PubMed
-
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. 2020. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181:271–280.E278. 10.1016/j.cell.2020.02.052. - DOI - PMC - PubMed
-
- Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V, Shawa I, Adams AC, Van Naarden J, Custer KL, Shen L, Durante M, Oakley G, Schade AE, Sabo J, Patel DR, Klekotka P, Skovronsky DM, BLAZE-1 Investigators. 2021. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med 384:229–237. 10.1056/NEJMoa2029849. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous