Ten years in the making: application of CrossMab technology for the development of therapeutic bispecific antibodies and antibody fusion proteins
- PMID: 34491877
- PMCID: PMC8425689
- DOI: 10.1080/19420862.2021.1967714
Ten years in the making: application of CrossMab technology for the development of therapeutic bispecific antibodies and antibody fusion proteins
Abstract
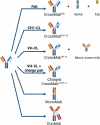
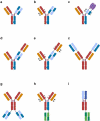
Bispecific antibodies have recently attracted intense interest. CrossMab technology was described in 2011 as novel approach enabling correct antibody light-chain association with their respective heavy chain in bispecific antibodies, together with methods enabling correct heavy-chain association using existing pairs of antibodies. Since the original description, CrossMab technology has evolved in the past decade into one of the most mature, versatile, and broadly applied technologies in the field, and nearly 20 bispecific antibodies based on CrossMab technology developed by Roche and others have entered clinical trials. The most advanced of these are the Ang-2/VEGF bispecific antibody faricimab, currently undergoing regulatory review, and the CD20/CD3 T cell bispecific antibody glofitamab, currently in pivotal Phase 3 trials. In this review, we introduce the principles of CrossMab technology, including its application for the generation of bi-/multispecific antibodies with different geometries and mechanisms of action, and provide an overview of CrossMab-based therapeutics in clinical trials.
Keywords: Bispecific; CrossMab; Immunotherapy; Oncology; Ophthalmology; T cell engager; TCB.
Conflict of interest statement
MS declares employment with Roche, WS declares patents/royalties with Roche and CK declares employment, stock ownership, and patents/royalties with Roche. CROSSMAB® is a registered trademark by Genentech/Roche.
Figures
Similar articles
-
The use of CrossMAb technology for the generation of bi- and multispecific antibodies.MAbs. 2016 Aug-Sep;8(6):1010-20. doi: 10.1080/19420862.2016.1197457. Epub 2016 Jun 10. MAbs. 2016. PMID: 27285945 Free PMC article. Review.
-
Engineering therapeutic bispecific antibodies using CrossMab technology.Methods. 2019 Feb 1;154:21-31. doi: 10.1016/j.ymeth.2018.11.008. Epub 2018 Nov 16. Methods. 2019. PMID: 30453028 Review.
-
Ang-2-VEGF-A CrossMab, a novel bispecific human IgG1 antibody blocking VEGF-A and Ang-2 functions simultaneously, mediates potent antitumor, antiangiogenic, and antimetastatic efficacy.Clin Cancer Res. 2013 Dec 15;19(24):6730-40. doi: 10.1158/1078-0432.CCR-13-0081. Epub 2013 Oct 4. Clin Cancer Res. 2013. PMID: 24097868
-
Progress in overcoming the chain association issue in bispecific heterodimeric IgG antibodies.MAbs. 2012 Nov-Dec;4(6):653-63. doi: 10.4161/mabs.21379. Epub 2012 Aug 27. MAbs. 2012. PMID: 22925968 Free PMC article. Review.
-
Generating bispecific human IgG1 and IgG2 antibodies from any antibody pair.J Mol Biol. 2012 Jul 13;420(3):204-19. doi: 10.1016/j.jmb.2012.04.020. Epub 2012 Apr 25. J Mol Biol. 2012. PMID: 22543237
Cited by
-
Adapting Neutralizing Antibodies to Viral Variants by Structure-Guided Affinity Maturation Using Phage Display Technology.Glob Chall. 2023 Aug 21;7(10):2300088. doi: 10.1002/gch2.202300088. eCollection 2023 Oct. Glob Chall. 2023. PMID: 37829677 Free PMC article.
-
Identification of tyrosine sulfation in the variable region of a bispecific antibody and its effect on stability and biological activity.MAbs. 2023 Jan-Dec;15(1):2259289. doi: 10.1080/19420862.2023.2259289. Epub 2023 Sep 24. MAbs. 2023. PMID: 37742207 Free PMC article.
-
Fifty years of monoclonals: the past, present and future of antibody therapeutics.Nat Rev Immunol. 2025 Aug 7. doi: 10.1038/s41577-025-01207-9. Online ahead of print. Nat Rev Immunol. 2025. PMID: 40775487 Review.
-
Immunotherapy of Multiple Myeloma: Current Status as Prologue to the Future.Int J Mol Sci. 2023 Oct 27;24(21):15674. doi: 10.3390/ijms242115674. Int J Mol Sci. 2023. PMID: 37958658 Free PMC article. Review.
-
Bispecific Antibody-Based Immune-Cell Engagers and Their Emerging Therapeutic Targets in Cancer Immunotherapy.Int J Mol Sci. 2022 May 19;23(10):5686. doi: 10.3390/ijms23105686. Int J Mol Sci. 2022. PMID: 35628495 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
