Murine norovirus virulence factor 1 (VF1) protein contributes to viral fitness during persistent infection
- PMID: 34491891
- PMCID: PMC8567427
- DOI: 10.1099/jgv.0.001651
Murine norovirus virulence factor 1 (VF1) protein contributes to viral fitness during persistent infection
Abstract
Murine norovirus (MNV) is widely used as a model for studying norovirus biology. While MNV isolates vary in their pathogenesis, infection of immunocompetent mice mostly results in persistent infection. The ability of a virus to establish a persistent infection is dependent on its ability to subvert or avoid the host immune response. Previously, we described the identification and characterization of virulence factor 1 (VF1) in MNV, and demonstrated its role as an innate immune antagonist. Here, we explore the role of VF1 during persistent MNV infection in an immunocompetent host. Using reverse genetics, we generated MNV-3 viruses carrying a single or a triple termination codon inserted in the VF1 ORF. VF1-deleted MNV-3 replicated to comparable levels to the wildtype virus in tissue culture. Comparative studies between MNV-3 and an acute MNV-1 strain show that MNV-3 VF1 exerts the same functions as MNV-1 VF1, but with reduced potency. C57BL/6 mice infected with VF1-deleted MNV-3 showed significantly reduced replication kinetics during the acute phase of the infection, but viral loads rapidly reached the levels seen in mice infected with wildtype virus after phenotypic restoration of VF1 expression. Infection with an MNV-3 mutant that had three termination codons inserted into VF1, in which reversion was suppressed, resulted in consistently lower replication throughout a 3 month persistent infection in mice, suggesting a role for VF1 in viral fitness in vivo. Our results indicate that VF1 expressed by a persistent strain of MNV also functions to antagonize the innate response to infection. We found that VF1 is not essential for viral persistence, but instead contributes to viral fitness in mice. These data fit with the hypothesis that noroviruses utilize multiple mechanisms to avoid and/or control the host response to infection and that VF1 is just one component of this.
Keywords: VF1; accessory protein; interferon response; norovirus; viral persistence.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures



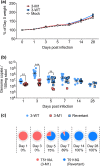
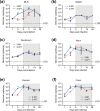
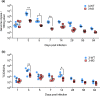
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

