Macrophage monocarboxylate transporter 1 promotes peripheral nerve regeneration after injury in mice
- PMID: 34491913
- PMCID: PMC8553554
- DOI: 10.1172/JCI141964
Macrophage monocarboxylate transporter 1 promotes peripheral nerve regeneration after injury in mice
Abstract
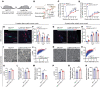
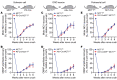
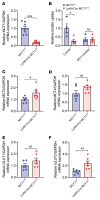
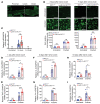
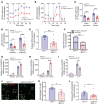
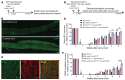
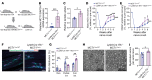
Peripheral nerves have the capacity for regeneration, but the rate of regeneration is so slow that many nerve injuries lead to incomplete recovery and permanent disability for patients. Macrophages play a critical role in the peripheral nerve response to injury, contributing to both Wallerian degeneration and nerve regeneration, and their function has recently been shown to be dependent on intracellular metabolism. To date, the impact of their intracellular metabolism on peripheral nerve regeneration has not been studied. We examined conditional transgenic mice with selective ablation in macrophages of solute carrier family 16, member 1 (Slc16a1), which encodes monocarboxylate transporter 1 (MCT1), and found that MCT1 contributed to macrophage metabolism, phenotype, and function, specifically in regard to phagocytosis and peripheral nerve regeneration. Adoptive cell transfer of wild-type macrophages ameliorated the impaired nerve regeneration in macrophage-selective MCT1-null mice. We also developed a mouse model that overexpressed MCT1 in macrophages and found that peripheral nerves in these mice regenerated more rapidly than in control mice. Our study provides further evidence that MCT1 has an important biological role in macrophages and that manipulations of macrophage metabolism can enhance recovery from peripheral nerve injuries, for which there are currently no approved medical therapies.
Keywords: Cytokines; Innervation; Macrophages; Metabolism; Neuroscience.
Conflict of interest statement
Figures

References
-
- Conforti L, et al. Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nat Rev Neurosci. 2014;15(6):394–409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

