Acute Lower Extremity Arterial Thrombosis Associated with Osimertinib-Induced Erythrocytosis
- PMID: 34491978
- PMCID: PMC8436826
- DOI: 10.12659/AJCR.932252
Acute Lower Extremity Arterial Thrombosis Associated with Osimertinib-Induced Erythrocytosis
Abstract
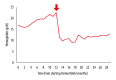
BACKGROUND Osimertinib is an oral third-generation epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) approved as first-line therapy for advanced non-small cell lung cancer (NSCLC) with positive EGFR mutation. Rashes, nail toxicity, and diarrhea are common adverse events. Hematological adverse effects, including anemia, thrombocytopenia, and lymphocytopenia, have been reported. However, erythrocytosis has not been reported as an adverse event. To the best of our knowledge, we report the first case of acute lower extremity thrombosis presumably caused by osimertinib-induced erythrocytosis. CASE REPORT A 70-year-old man with epidermal EGFR-mutant advanced NSCLC presented with acute left sural pain. The patient's left foot was cold, and peripheral arterial Doppler signals were absent. He had developed erythrocytosis of unknown etiology during osimertinib therapy. Hemoglobin (Hb) and hematocrit were 22.6 g/dL and 62.5%, respectively. Contrast-enhanced computed tomography showed thrombotic occlusion of the popliteal artery. Other than erythrocytosis, there was no possible cause of arterial thrombosis. Osimertinib was discontinued immediately because the NSCLC started to resist treatment and was presumed to be the cause of erythrocytosis. He received endovascular treatment (EVT). Following serial EVT and debridement, his fourth toe was amputated for necrosis. Erythrocytosis persisted 8 months during osimertinib therapy. Hb levels decreased to 15.4 mg/dL due to blood loss complicated with catheter thrombectomy and remained normal for 20 months after osimertinib discontinuation. The patient died of cancer progression. CONCLUSIONS This case suggests the erythrocytosis was possibly caused by osimertinib. We may need to monitor Hb levels during osimertinib therapy and be alert to thrombosis once Hb starts to rise.
Conflict of interest statement
None declared.
Figures
References
-
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–25. - PubMed
-
- Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer. Ann Oncol. 2015;26:1877. - PubMed
-
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J Clin Oncol. 2011;29:2866. - PubMed
-
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemo-therapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous