ASCENT (Automated Simulations to Characterize Electrical Nerve Thresholds): A pipeline for sample-specific computational modeling of electrical stimulation of peripheral nerves
- PMID: 34492004
- PMCID: PMC8423288
- DOI: 10.1371/journal.pcbi.1009285
ASCENT (Automated Simulations to Characterize Electrical Nerve Thresholds): A pipeline for sample-specific computational modeling of electrical stimulation of peripheral nerves
Abstract
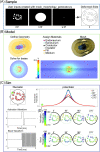
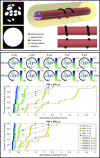
Electrical stimulation and block of peripheral nerves hold great promise for treatment of a range of disease and disorders, but promising results from preclinical studies often fail to translate to successful clinical therapies. Differences in neural anatomy across species require different electrodes and stimulation parameters to achieve equivalent nerve responses, and accounting for the consequences of these factors is difficult. We describe the implementation, validation, and application of a standardized, modular, and scalable computational modeling pipeline for biophysical simulations of electrical activation and block of nerve fibers within peripheral nerves. The ASCENT (Automated Simulations to Characterize Electrical Nerve Thresholds) pipeline provides a suite of built-in capabilities for user control over the entire workflow, including libraries for parts to assemble electrodes, electrical properties of biological materials, previously published fiber models, and common stimulation waveforms. We validated the accuracy of ASCENT calculations, verified usability in beta release, and provide several compelling examples of ASCENT-implemented models. ASCENT will enable the reproducibility of simulation data, and it will be used as a component of integrated simulations with other models (e.g., organ system models), to interpret experimental results, and to design experimental and clinical interventions for the advancement of peripheral nerve stimulation therapies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Scaling of vagus nerve stimulation parameters does not achieve equivalent nerve responses across species.Bioelectron Med. 2025 May 16;11(1):11. doi: 10.1186/s42234-025-00174-9. Bioelectron Med. 2025. PMID: 40375300 Free PMC article.
-
Ultrasound guidance for upper and lower limb blocks.Cochrane Database Syst Rev. 2015 Sep 11;2015(9):CD006459. doi: 10.1002/14651858.CD006459.pub3. Cochrane Database Syst Rev. 2015. PMID: 26361135 Free PMC article.
-
A study of flex miniaturized coils for focal nerve magnetic stimulation.Med Phys. 2023 Mar;50(3):1779-1792. doi: 10.1002/mp.16148. Epub 2022 Dec 29. Med Phys. 2023. PMID: 36502488 Free PMC article.
-
Transcutaneous electrical nerve stimulation (TENS) for dementia.Cochrane Database Syst Rev. 2003;2003(3):CD004032. doi: 10.1002/14651858.CD004032. Cochrane Database Syst Rev. 2003. PMID: 12917999 Free PMC article.
-
Bioengineered nerve conduits and wraps for peripheral nerve repair of the upper limb.Cochrane Database Syst Rev. 2022 Dec 7;12(12):CD012574. doi: 10.1002/14651858.CD012574.pub2. Cochrane Database Syst Rev. 2022. PMID: 36477774 Free PMC article.
Cited by
-
A computational roadmap to electronic drugs.Front Neurorobot. 2022 Oct 31;16:983072. doi: 10.3389/fnbot.2022.983072. eCollection 2022. Front Neurorobot. 2022. PMID: 36386388 Free PMC article.
-
Guidance for sharing computational models of neural stimulation: from project planning to publication.J Neural Eng. 2025 Mar 13;22(2):10.1088/1741-2552/adb997. doi: 10.1088/1741-2552/adb997. J Neural Eng. 2025. PMID: 39993327 Free PMC article. Review.
-
Towards enhanced functionality of vagus neuroprostheses through in silico optimized stimulation.Nat Commun. 2024 Jul 20;15(1):6119. doi: 10.1038/s41467-024-50523-6. Nat Commun. 2024. PMID: 39033186 Free PMC article.
-
AxoDetect: an automated nerve image segmentation and quantification workflow for computational nerve modeling.J Neural Eng. 2024 Mar 28;21(2):026017. doi: 10.1088/1741-2552/ad31c3. J Neural Eng. 2024. PMID: 38457836 Free PMC article.
-
Advanced neuroprosthetic electrode design optimized by electromagnetic finite element simulation: innovations and applications.Front Bioeng Biotechnol. 2024 Nov 6;12:1476447. doi: 10.3389/fbioe.2024.1476447. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39574462 Free PMC article. Review.
References
-
- Ben-Menachem E, Manon-Espaillat R, Ristanovic R, Wilder BJ, Stefan H, Mirza W, et al.. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35(3):616–26. Available from: doi: 10.1111/j.1528-1157.1994.tb02482.x - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

