The cardiovascular effects of amodiaquine and structurally related antimalarials: An individual patient data meta-analysis
- PMID: 34492005
- PMCID: PMC8454971
- DOI: 10.1371/journal.pmed.1003766
The cardiovascular effects of amodiaquine and structurally related antimalarials: An individual patient data meta-analysis
Abstract
Background: Amodiaquine is a 4-aminoquinoline antimalarial similar to chloroquine that is used extensively for the treatment and prevention of malaria. Data on the cardiovascular effects of amodiaquine are scarce, although transient effects on cardiac electrophysiology (electrocardiographic QT interval prolongation and sinus bradycardia) have been observed. We conducted an individual patient data meta-analysis to characterise the cardiovascular effects of amodiaquine and thereby support development of risk minimisation measures to improve the safety of this important antimalarial.
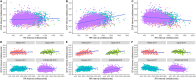
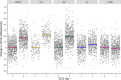
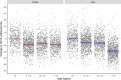
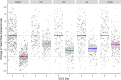
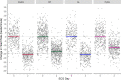
Methods and findings: Studies of amodiaquine for the treatment or prevention of malaria were identified from a systematic review. Heart rates and QT intervals with study-specific heart rate correction (QTcS) were compared within studies and individual patient data pooled for multivariable linear mixed effects regression. The meta-analysis included 2,681 patients from 4 randomised controlled trials evaluating artemisinin-based combination therapies (ACTs) containing amodiaquine (n = 725), lumefantrine (n = 499), piperaquine (n = 716), and pyronaridine (n = 566), as well as monotherapy with chloroquine (n = 175) for uncomplicated malaria. Amodiaquine prolonged QTcS (mean = 16.9 ms, 95% CI: 15.0 to 18.8) less than chloroquine (21.9 ms, 18.3 to 25.6, p = 0.0069) and piperaquine (19.2 ms, 15.8 to 20.5, p = 0.0495), but more than lumefantrine (5.6 ms, 2.9 to 8.2, p < 0.001) and pyronaridine (-1.2 ms, -3.6 to +1.3, p < 0.001). In individuals aged ≥12 years, amodiaquine reduced heart rate (mean reduction = 15.2 beats per minute [bpm], 95% CI: 13.4 to 17.0) more than piperaquine (10.5 bpm, 7.7 to 13.3, p = 0.0013), lumefantrine (9.3 bpm, 6.4 to 12.2, p < 0.001), pyronaridine (6.6 bpm, 4.0 to 9.3, p < 0.001), and chloroquine (5.9 bpm, 3.2 to 8.5, p < 0.001) and was associated with a higher risk of potentially symptomatic sinus bradycardia (≤50 bpm) than lumefantrine (risk difference: 14.8%, 95% CI: 5.4 to 24.3, p = 0.0021) and chloroquine (risk difference: 8.0%, 95% CI: 4.0 to 12.0, p < 0.001). The effect of amodiaquine on the heart rate of children aged <12 years compared with other antimalarials was not clinically significant. Study limitations include the unavailability of individual patient-level adverse event data for most included participants, but no serious complications were documented.
Conclusions: While caution is advised in the use of amodiaquine in patients aged ≥12 years with concomitant use of heart rate-reducing medications, serious cardiac conduction disorders, or risk factors for torsade de pointes, there have been no serious cardiovascular events reported after amodiaquine in widespread use over 7 decades. Amodiaquine and structurally related antimalarials in the World Health Organization (WHO)-recommended dose regimens alone or in ACTs are safe for the treatment and prevention of malaria.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: EAA and NJW are members of the Editorial Board of PLOS Medicine.
Figures
References
-
- World Health Organization. World Malaria Report 2019. Geneva, Switzerland: 2019.
-
- World Health Organization. Guidelines for the Treatment of Malaria. 3rd ed. Geneva, Switzerland: 2015.
-
- World Health Organization. Seasonal malaria chemoprevention with sulfadoxine-pyrimethamine plus amodiaquine in children: A field guide. Geneva, Switzerland: 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

