Ascension of Chlamydia is moderated by uterine peristalsis and the neutrophil response to infection
- PMID: 34492008
- PMCID: PMC8448331
- DOI: 10.1371/journal.pcbi.1009365
Ascension of Chlamydia is moderated by uterine peristalsis and the neutrophil response to infection
Abstract
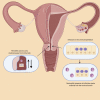
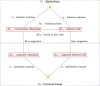
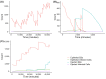
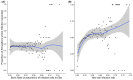
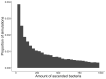
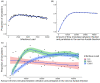
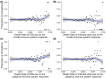
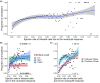
Chlamydia trachomatis is a common sexually transmitted infection that is associated with a range of serious reproductive tract sequelae including in women Pelvic Inflammatory Disease (PID), tubal factor infertility, and ectopic pregnancy. Ascension of the pathogen beyond the cervix and into the upper reproductive tract is thought to be necessary for these pathologies. However, Chlamydia trachomatis does not encode a mechanism for movement on its genome, and so the processes that facilitate ascension have not been elucidated. Here, we evaluate the factors that may influence chlamydial ascension in women. We constructed a mathematical model based on a set of stochastic dynamics to elucidate the moderating factors that might influence ascension of infections in the first month of an infection. In the simulations conducted from the stochastic model, 36% of infections ascended, but only 9% had more than 1000 bacteria ascend. The results of the simulations indicated that infectious load and the peristaltic contractions moderate ascension and are inter-related in impact. Smaller initial loads were much more likely to ascend. Ascension was found to be dependent on the neutrophil response. Overall, our results indicate that infectious load, menstrual cycle timing, and the neutrophil response are critical factors in chlamydial ascension in women.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Chlamydia trachomatis: impact on human reproduction.Hum Reprod Update. 1999 Sep-Oct;5(5):433-47. doi: 10.1093/humupd/5.5.433. Hum Reprod Update. 1999. PMID: 10582782 Review.
-
Acute pelvic inflammatory disease.Urol Clin North Am. 1984 Feb;11(1):65-81. Urol Clin North Am. 1984. PMID: 6369707 Review.
-
Risk of Ectopic Pregnancy and Tubal Infertility Following Gonorrhea and Chlamydia Infections.Clin Infect Dis. 2019 Oct 15;69(9):1621-1623. doi: 10.1093/cid/ciz145. Clin Infect Dis. 2019. PMID: 30778532
-
Chlamydial infection and spatial ascension of the female genital tract: a novel hybrid cellular automata and continuum mathematical model.FEMS Immunol Med Microbiol. 2009 Nov;57(2):173-82. doi: 10.1111/j.1574-695X.2009.00596.x. Epub 2009 Aug 12. FEMS Immunol Med Microbiol. 2009. PMID: 19735471 Free PMC article.
-
Residues of pelvic inflammatory disease in intrauterine device users: a result of the intrauterine device or Chlamydia trachomatis infection?Am J Obstet Gynecol. 1986 Mar;154(3):497-503. doi: 10.1016/0002-9378(86)90590-9. Am J Obstet Gynecol. 1986. PMID: 3513579
Cited by
-
A 5-HT-mediated urethral defense against urinary tract infections.Proc Natl Acad Sci U S A. 2025 Apr 22;122(16):e2409754122. doi: 10.1073/pnas.2409754122. Epub 2025 Apr 14. Proc Natl Acad Sci U S A. 2025. PMID: 40228121
-
Study Models for Chlamydia trachomatis Infection of the Female Reproductive Tract.Microorganisms. 2025 Feb 28;13(3):553. doi: 10.3390/microorganisms13030553. Microorganisms. 2025. PMID: 40142446 Free PMC article. Review.
-
Vaginal and tumor microbiomes in gynecological cancer (Review).Oncol Lett. 2023 Mar 3;25(4):153. doi: 10.3892/ol.2023.13739. eCollection 2023 Apr. Oncol Lett. 2023. PMID: 36936020 Free PMC article. Review.
-
Influence of cervicovaginal microbiota on Chlamydia trachomatis infection dynamics.Microb Cell. 2025 Apr 15;12:93-108. doi: 10.15698/mic2025.04.848. eCollection 2025. Microb Cell. 2025. PMID: 40309355 Free PMC article. Review.
-
IL-21/IL-21R Regulates the Neutrophil-Mediated Pathologic Immune Response during Chlamydial Respiratory Infection.Mediators Inflamm. 2022 Jun 1;2022:4322092. doi: 10.1155/2022/4322092. eCollection 2022. Mediators Inflamm. 2022. PMID: 35693111 Free PMC article.
References
-
- Low N, Egger M, Sterne JAC, Harbord RM, Ibrahim F, Lindblom B, et al.. Incidence of severe reproductive tract complications associated with diagnosed genital chlamydial infection: the Uppsala Women’s Cohort Study. Sex Transm Infect. 2006;82(3):212–8. doi: 10.1136/sti.2005.017186 - DOI - PMC - PubMed
-
- Westrom L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic Inflammatory Disease and Fertility. Sexually Transmitted Diseases. 1992;19(4):185–92. - PubMed
-
- Stacey C, Munday P, Thomas B, Gilchrist C, Taylor-Robinson D, Beard R. Chlamydia trachomatis in the fallopian tubes of women without laparoscopic evidence of salpingitis. International Journal of Gynecology & Obstetrics. 1991;35(3):287. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

