Comparison of antigen- and RT-PCR-based testing strategies for detection of SARS-CoV-2 in two high-exposure settings
- PMID: 34492025
- PMCID: PMC8423454
- DOI: 10.1371/journal.pone.0253407
Comparison of antigen- and RT-PCR-based testing strategies for detection of SARS-CoV-2 in two high-exposure settings
Abstract
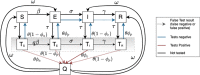
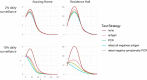
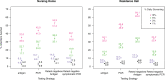
Surveillance testing for infectious disease is an important tool to combat disease transmission at the population level. During the SARS-CoV-2 pandemic, RT-PCR tests have been considered the gold standard due to their high sensitivity and specificity. However, RT-PCR tests for SARS-CoV-2 have been shown to return positive results when performed to individuals who are past the infectious stage of the disease. Meanwhile, antigen-based tests are often treated as a less accurate substitute for RT-PCR, however, new evidence suggests they may better reflect infectiousness. Consequently, the two test types may each be most optimally deployed in different settings. Here, we present an epidemiological model with surveillance testing and coordinated isolation in two congregate living settings (a nursing home and a university dormitory system) that considers test metrics with respect to viral culture, a proxy for infectiousness. Simulations show that antigen-based surveillance testing coupled with isolation greatly reduces disease burden and carries a lower economic cost than RT-PCR-based strategies. Antigen and RT-PCR tests perform different functions toward the goal of reducing infectious disease burden and should be used accordingly.
Conflict of interest statement
MTW, AC, DM, and CKC reported being employed by Becton, Dickinson, and Company. JL, DJAT, MHS, and LK received research funding from Becton, Dickinson, and Company for this study. In addition to funding listed in the funding statement, JL and DJAT have received research support from Pfizer, Inc. for unrelated work. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

