Dynamin inhibition causes context-dependent cell death of leukemia and lymphoma cells
- PMID: 34492077
- PMCID: PMC8423305
- DOI: 10.1371/journal.pone.0256708
Dynamin inhibition causes context-dependent cell death of leukemia and lymphoma cells
Abstract
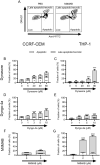
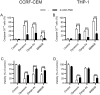
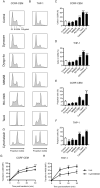
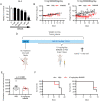
Current chemotherapy for treatment of pediatric acute leukemia, although generally successful, is still a matter of concern due to treatment resistance, relapses and life-long side effects for a subset of patients. Inhibition of dynamin, a GTPase involved in clathrin-mediated endocytosis and regulation of the cell cycle, has been proposed as a potential anti-cancer regimen, but the effects of dynamin inhibition on leukemia cells has not been extensively addressed. Here we adopted single cell and whole-population analysis by flow cytometry and live imaging, to assess the effect of dynamin inhibition (Dynasore, Dyngo-4a, MitMAB) on pediatric acute leukemia cell lines (CCRF-CEM and THP-1), human bone marrow biopsies from patients diagnosed with acute lymphoblastic leukemia (ALL), as well as in a model of lymphoma (EL4)-induced tumor growth in mice. All inhibitors suppressed proliferation and induced pronounced caspase-dependent apoptotic cell death in CCRF-CEM and THP-1 cell lines. However, the inhibitors showed no effect on bone marrow biopsies, and did not prevent EL4-induced tumor formation in mice. We conclude that dynamin inhibition affects highly proliferating human leukemia cells. These findings form a basis for evaluation of the potential, and constraints, of employing dynamin inhibition in treatment strategies against leukemia and other malignancies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Building a better dynasore: the dyngo compounds potently inhibit dynamin and endocytosis.Traffic. 2013 Dec;14(12):1272-89. doi: 10.1111/tra.12119. Epub 2013 Oct 9. Traffic. 2013. PMID: 24025110 Free PMC article.
-
Chemical Inhibitors of Dynamin Exert Differential Effects in VEGF Signaling.Cells. 2021 Apr 23;10(5):997. doi: 10.3390/cells10050997. Cells. 2021. PMID: 33922806 Free PMC article.
-
Beauvericin induces cytotoxic effects in human acute lymphoblastic leukemia cells through cytochrome c release, caspase 3 activation: the causative role of calcium.Cancer Lett. 2004 Dec 28;216(2):165-73. doi: 10.1016/j.canlet.2004.06.005. Cancer Lett. 2004. PMID: 15533592
-
Use of dynasore, the small molecule inhibitor of dynamin, in the regulation of endocytosis.Methods Enzymol. 2008;438:77-93. doi: 10.1016/S0076-6879(07)38006-3. Methods Enzymol. 2008. PMID: 18413242 Free PMC article.
-
Dynasore - not just a dynamin inhibitor.Cell Commun Signal. 2015 Apr 10;13:24. doi: 10.1186/s12964-015-0102-1. Cell Commun Signal. 2015. PMID: 25889964 Free PMC article. Review.
Cited by
-
Infection of liver sinusoidal endothelial cells with Muromegalovirus muridbeta1 involves binding to neuropilin-1 and is dynamin-dependent.Front Cell Infect Microbiol. 2023 Nov 9;13:1249894. doi: 10.3389/fcimb.2023.1249894. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38029264 Free PMC article.
-
The sulfonadyns: a class of aryl sulfonamides inhibiting dynamin I GTPase and clathrin mediated endocytosis are anti-seizure in animal models.RSC Med Chem. 2023 Apr 26;14(8):1492-1511. doi: 10.1039/d2md00371f. eCollection 2023 Aug 16. RSC Med Chem. 2023. PMID: 37593570 Free PMC article.
-
Small molecules targeting endocytic uptake and recycling pathways.Front Cell Dev Biol. 2023 Mar 10;11:1125801. doi: 10.3389/fcell.2023.1125801. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36968200 Free PMC article. Review.
-
Cancer as a biophysical disease: Targeting the mechanical-adaptability program.Biophys J. 2022 Oct 4;121(19):3573-3585. doi: 10.1016/j.bpj.2022.04.039. Epub 2022 May 3. Biophys J. 2022. PMID: 35505610 Free PMC article. Review.
-
Prodrugs of the Archetypal Dynamin Inhibitor Bis-T-22.ChemMedChem. 2022 Dec 16;17(24):e202200400. doi: 10.1002/cmdc.202200400. Epub 2022 Nov 9. ChemMedChem. 2022. PMID: 36351775 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

