Hepatitis C virus induces oxidation and degradation of apolipoprotein B to enhance lipid accumulation and promote viral production
- PMID: 34492079
- PMCID: PMC8448335
- DOI: 10.1371/journal.ppat.1009889
Hepatitis C virus induces oxidation and degradation of apolipoprotein B to enhance lipid accumulation and promote viral production
Abstract
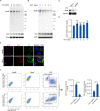
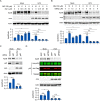
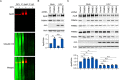
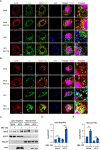
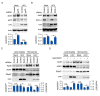
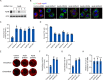
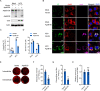
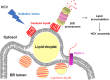
Hepatitis C virus (HCV) infection induces the degradation and decreases the secretion of apolipoprotein B (ApoB). Impaired production and secretion of ApoB-containing lipoprotein is associated with an increase in hepatic steatosis. Therefore, HCV infection-induced degradation of ApoB may contribute to hepatic steatosis and decreased lipoprotein secretion, but the mechanism of HCV infection-induced ApoB degradation has not been completely elucidated. In this study, we found that the ApoB level in HCV-infected cells was regulated by proteasome-associated degradation but not autophagic degradation. ApoB was degraded by the 20S proteasome in a ubiquitin-independent manner. HCV induced the oxidation of ApoB via oxidative stress, and oxidized ApoB was recognized by the PSMA5 and PSMA6 subunits of the 20S proteasome for degradation. Further study showed that ApoB was degraded at endoplasmic reticulum (ER)-associated lipid droplets (LDs) and that the retrotranslocation and degradation of ApoB required Derlin-1 but not gp78 or p97. Moreover, we found that knockdown of ApoB before infection increased the cellular lipid content and enhanced HCV assembly. Overexpression of ApoB-50 inhibited lipid accumulation and repressed viral assembly in HCV-infected cells. Our study reveals a novel mechanism of ApoB degradation and lipid accumulation during HCV infection and might suggest new therapeutic strategies for hepatic steatosis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

