Prenatal alcohol exposure disrupts Sonic hedgehog pathway and primary cilia genes in the mouse neural tube
- PMID: 34492310
- PMCID: PMC8529623
- DOI: 10.1016/j.reprotox.2021.09.002
Prenatal alcohol exposure disrupts Sonic hedgehog pathway and primary cilia genes in the mouse neural tube
Abstract
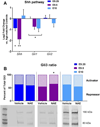
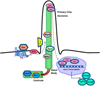
Neurulation-stage alcohol exposure (NAE; embryonic day [E] 8-10) is associated with midline craniofacial and CNS defects that likely arise from disruption of morphogen pathways, such as Sonic hedgehog (Shh). Notably, midline anomalies are also a hallmark of genetic ciliopathies such as Joubert syndrome. We tested whether NAE alters Shh pathway signaling and the number and function of primary cilia, organelles critical for Shh pathway transduction. Female C57BL/6 J mice were administered two doses of alcohol (2.9 g/kg/dose) or vehicle on E9. Embryos were collected 6, 12, or 24 h later, and changes to Shh, cell cycle genes, and primary cilia were measured in the rostroventral neural tube (RVNT). Within the first 24 h post-NAE, reductions in Shh pathway and cell cycle gene expression and the ratio of Gli3 forms in the full-length activator state were observed. RVNT volume and cell layer width were reduced at 12 h. In addition, altered expression of multiple cilia-related genes was observed at 6 h post-NAE. As a further test of cilia gene-ethanol interaction, mice heterozygous for Kif3a exhibited perturbed behavior during adolescence following NAE compared to vehicle-treated mice, and Kif3a heterozygosity exacerbated the hyperactive effects of NAE on exploratory activity. These data demonstrate that NAE downregulates the Shh pathway in a region of the neural tube that gives rise to alcohol-sensitive brain structures and identifies disruption of primary cilia function, or a "transient ciliopathy", as a possible cellular mechanism of prenatal alcohol pathogenesis.
Keywords: Cell cycle; Development; Fetal alcohol spectrum disorders; Kif3a; Neurulation.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Competing interests
The authors have no conflicts of interest to report.
Figures



References
-
- Cook CS, Nowotny AZ, Sulik KK, Fetal alcohol syndrome: eye malformations in a mouse model, Archives of Ophthalmology 105(11) (1987) 1576–1581. - PubMed
-
- Godin EA, O’Leary Moore SK, Khan AA, Parnell SE, Ament JJ, Dehart DB, Johnson BW, Allan Johnson G, Styner MA, Sulik KK, Magnetic Resonance Microscopy Defines Ethanol Induced Brain Abnormalities in Prenatal Mice: Effects of Acute Insult on Gestational Day 7, Alcoholism: Clinical and Experimental Research 34(1) (2010) 98–111. - PMC - PubMed
-
- Dunty WC, Chen S.y., Zucker RM, Dehart DB, Sulik KK, Selective Vulnerability of Embryonic Cell Populations to Ethanol Induced Apoptosis: Implications for Alcohol Related Birth Defects and Neurodevelopmental Disorder, Alcoholism: Clinical and Experimental Research 25(10) (2001) 1523–1535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

