Interrogating the relationship between the microstructure of amphiphilic poly(ethylene glycol-b-caprolactone) copolymers and their colloidal assemblies using non-interfering techniques
- PMID: 34492457
- PMCID: PMC8645339
- DOI: 10.1016/j.jcis.2021.08.084
Interrogating the relationship between the microstructure of amphiphilic poly(ethylene glycol-b-caprolactone) copolymers and their colloidal assemblies using non-interfering techniques
Abstract
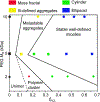
Understanding the microstructural parameters of amphiphilic copolymers that control the formation and structure of aggregated colloids (e.g., micelles) is essential for the rational design of hierarchically structured systems for applications in nanomedicine, personal care and food formulations. Although many analytical techniques have been employed to study such systems, in this investigation we adopted an integrated approach using non-interfering techniques - diffusion nuclear magnetic resonance (NMR) spectroscopy, dynamic light scattering (DLS) and synchrotron small-angle X-ray scattering (SAXS) - to probe the relationship between the microstructure of poly(ethylene glycol-b-caprolactone) (PEG-b-PCL) copolymers [e.g., block molecular weight (MW) and the mass fraction of PCL (fPCL)] and the structure of their aggregates. Systematic trends in the self-assembly behaviour were determined using a large family of well-defined block copolymers with variable PEG and PCL block lengths (number-average molecular weights (Mn) between 2 and 10 and 0.5-15 kDa, respectively) and narrow dispersity (Ð < 1.12). For all of the copolymers, a clear transition in the aggregate structure was observed when the hydrophobic fPCL was increased at a constant PEG block Mn, although the nature of this transition is also dependent on the PEG block Mn. Copolymers with low Mn PEG blocks (2 kDa) were observed to transition from unimers and loosely associated unimers to metastable aggregates and finally, to cylindrical micelles as the fPCL was increased. In comparison, copolymers with PEG block Mn of between 5 and 10 kDa transitioned from heterogenous metastable aggregates to cylindrical micelles and finally, well-defined ellipsoidal micelles (of decreasing aspect ratios) as the fPCL was increased. In all cases, the diffusion NMR spectroscopy, DLS and synchrotron SAXS results provided complementary information and the grounds for a phase diagram relating copolymer microstructure to aggregation behaviour and structure. Importantly, the absence of commonly depicted spherical micelles has implications for applications where properties may be governed by shape, such as, cellular uptake of nanomedicine formulations.
Keywords: Aggregate; Aggregated colloid; Diffusion NMR spectroscopy; Micelle; Microstructure; Poly(ethylene glycol-b-caprolactone) copolymers; Self-assembly; Small angle X-ray scattering.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Kim T-Y; Kim D-W; Chung J-Y; Shin SG; Kim S-C; Heo DS; Kim NK; Bang Y-J, Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin. Cancer Res 2004, 10 (11), 3708. doi: 10.1158/1078-0432.CCR-03-0655. - DOI - PubMed
-
- AIHW Cancer in Australia 2010: an overview http://www.aihw.gov.au/publication-detail/?id=6442472459 (accessed 25/05/2015).
-
- Hamaguchi T; Kato K; Yasui H; Morizane C; Ikeda M; Ueno H; Muro K; Yamada Y; Okusaka T; Shirao K; Shimada Y; Nakahama H; Matsumura Y, A phase I and pharmacokinetic study of NK105, a paclitaxel-incorporating micellar nanoparticle formulation. Br. J. Cancer 2007, 97 (2), 170–6, doi: 10.1038/sj.bjc.6603855. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

