Flow radiocytometry using droplet optofluidics
- PMID: 34492500
- PMCID: PMC8530933
- DOI: 10.1016/j.bios.2021.113565
Flow radiocytometry using droplet optofluidics
Abstract
Flow-based cytometry methods are widely used to analyze heterogeneous cell populations. However, their use for small molecule studies remains limited due to bulky fluorescent labels that often interfere with biochemical activity in cells. In contrast, radiotracers require minimal modification of their target molecules and can track biochemical processes with negligible interference and high specificity. Here, we introduce flow radiocytometry (FRCM) that broadens the scope of current cytometry methods to include beta-emitting radiotracers as probes for single cell studies. FRCM uses droplet microfluidics and radiofluorogenesis to translate the radioactivity of single cells into a fluorescent signal that is then read out using a high-throughput optofluidic device. As a proof of concept, we quantitated [18F]fluorodeoxyglucose radiotracer uptake in single human breast cancer cells and successfully assessed the metabolic flux of glucose and its heterogeneity at the cellular level. We believe FRCM has potential applications ranging from analytical assays for cancer and other diseases to development of small-molecule drugs.
Keywords: Droplet microfluidics; Fluorodeoxyglucose; Optofluidics; Radiochemistry; Radiofluorogenesis; Single-cell analysis.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
COMPETING INTERESTS
G.P. is listed as inventor on a patent (US 20160025701 A1) related to this work. Other authors declare no conflict of interest.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
G.P. is listed as inventor on a patent (US 20160025701 A1) related to this work. Other authors declare no conflict of interest.
Figures
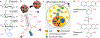

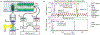



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

