Core set of patient-reported outcomes for myelodysplastic syndromes: an EUMDS Delphi study involving patients and hematologists
- PMID: 34492684
- PMCID: PMC8753222
- DOI: 10.1182/bloodadvances.2021004568
Core set of patient-reported outcomes for myelodysplastic syndromes: an EUMDS Delphi study involving patients and hematologists
Abstract
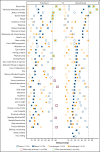
Patient-reported outcomes (PROs) are relevant and valuable end points in the care of patients with myelodysplastic syndromes (MDS). However, a consensus-based selection of PROs for MDS, derived by both patients and hematologists, is lacking. We aimed to develop a core set of PROs for patients with MDS as part of the prospective European LeukemiaNet MDS (EUMDS) Registry. According to international guidelines, candidate PROs were identified from a comprehensive literature search of MDS studies. Overall, 40 PROs were selected and evaluated in a two-round Delphi survey by 40 patients with MDS and 38 hematologists in the first round and 38 patients and 32 hematologists in the second round. Based on an agreement scale and predefined inclusion criteria, both patients and hematologists selected "general quality of life" as a core PRO. Hematologists also selected "transfusion-dependency burden" and "ability to work/activities of daily living" as core PROs. The second Delphi round increased PRO rating agreements. Statistically significant rating differences between patients and hematologists were observed for 28 PROs (Mann-Whitney U test; P < .05) in the first round and for 19 PROs in the second round, with "disease knowledge" and "confidence in health care services" rated notably higher by patients. The overall mean PRO ratings correlation between the 2 groups was moderate (Spearman's rank correlation coefficient = 0.5; P < .05). This first consensus on a core set of PROs jointly developed by patients and hematologists forms the basis for patient-centered care in daily practice and clinical research.
© 2021 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures

References
-
- Siebert U. When should decision-analytic modeling be used in the economic evaluation of health care? Eur J Health Econ. 2003;4(3):143-150.
-
- Cheson BD, Bennett JM, Kantarjian H, et al. ; World Health Organization(WHO) international working group . Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96(12):3671-3674. - PubMed
-
- Patel SS, Gerds AT. Patient-reported outcomes in myelodysplastic syndromes and MDS/MPN overlap syndromes: stepping onto the stage with changing times. Curr Hematol Malig Rep. 2017;12(5):455-460. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

