The immunometabolite itaconate inhibits heme synthesis and remodels cellular metabolism in erythroid precursors
- PMID: 34492704
- PMCID: PMC9153040
- DOI: 10.1182/bloodadvances.2021004750
The immunometabolite itaconate inhibits heme synthesis and remodels cellular metabolism in erythroid precursors
Abstract
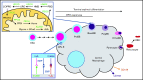
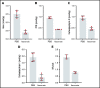
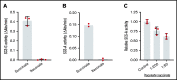
As part of the inflammatory response by macrophages, Irg1 is induced, resulting in millimolar quantities of itaconate being produced. This immunometabolite remodels the macrophage metabolome and acts as an antimicrobial agent when excreted. Itaconate is not synthesized within the erythron but instead may be acquired from central macrophages within the erythroid island. Previously, we reported that itaconate inhibits hemoglobinization of developing erythroid cells. Herein we show that this action is accomplished by inhibition of tetrapyrrole synthesis. In differentiating erythroid precursors, cellular heme and protoporphyrin IX synthesis are reduced by itaconate at an early step in the pathway. In addition, itaconate causes global alterations in cellular metabolite pools, resulting in elevated levels of succinate, 2-hydroxyglutarate, pyruvate, glyoxylate, and intermediates of glycolytic shunts. Itaconate taken up by the developing erythron can be converted to itaconyl-coenzyme A (CoA) by the enzyme succinyl-CoA:glutarate-CoA transferase. Propionyl-CoA, propionyl-carnitine, methylmalonic acid, heptadecanoic acid, and nonanoic acid, as well as the aliphatic amino acids threonine, valine, methionine, and isoleucine, are increased, likely due to the impact of endogenous itaconyl-CoA synthesis. We further show that itaconyl-CoA is a competitive inhibitor of the erythroid-specific 5-aminolevulinate synthase (ALAS2), the first and rate-limiting step in heme synthesis. These findings strongly support our hypothesis that the inhibition of heme synthesis observed in chronic inflammation is mediated not only by iron limitation but also by limitation of tetrapyrrole synthesis at the point of ALAS2 catalysis by itaconate. Thus, we propose that macrophage-derived itaconate promotes anemia during an inflammatory response in the erythroid compartment.
© 2021 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures





Similar articles
-
Abolition of mitochondrial substrate-level phosphorylation by itaconic acid produced by LPS-induced Irg1 expression in cells of murine macrophage lineage.FASEB J. 2016 Jan;30(1):286-300. doi: 10.1096/fj.15-279398. Epub 2015 Sep 10. FASEB J. 2016. PMID: 26358042
-
Glutamine via α-ketoglutarate dehydrogenase provides succinyl-CoA for heme synthesis during erythropoiesis.Blood. 2018 Sep 6;132(10):987-998. doi: 10.1182/blood-2018-01-829036. Epub 2018 Jul 10. Blood. 2018. PMID: 29991557 Free PMC article.
-
Itaconate Alters Succinate and Coenzyme A Metabolism via Inhibition of Mitochondrial Complex II and Methylmalonyl-CoA Mutase.Metabolites. 2021 Feb 18;11(2):117. doi: 10.3390/metabo11020117. Metabolites. 2021. PMID: 33670656 Free PMC article.
-
Metabolite itaconate in host immunoregulation and defense.Cell Mol Biol Lett. 2023 Dec 2;28(1):100. doi: 10.1186/s11658-023-00503-3. Cell Mol Biol Lett. 2023. PMID: 38042791 Free PMC article. Review.
-
Itaconate: an emerging determinant of inflammation in activated macrophages.Immunol Cell Biol. 2019 Feb;97(2):134-141. doi: 10.1111/imcb.12218. Epub 2018 Dec 11. Immunol Cell Biol. 2019. PMID: 30428148 Review.
Cited by
-
New Avenues of Heme Synthesis Regulation.Int J Mol Sci. 2022 Jul 5;23(13):7467. doi: 10.3390/ijms23137467. Int J Mol Sci. 2022. PMID: 35806474 Free PMC article. Review.
-
Itaconate: A Potent Macrophage Immunomodulator.Inflammation. 2023 Aug;46(4):1177-1191. doi: 10.1007/s10753-023-01819-0. Epub 2023 May 4. Inflammation. 2023. PMID: 37142886 Free PMC article. Review.
-
Protective role of aconitate decarboxylase 1 in neuroinflammation-induced dysfunctions of the paraventricular thalamus and sleepiness.Commun Biol. 2024 Nov 10;7(1):1484. doi: 10.1038/s42003-024-07215-0. Commun Biol. 2024. PMID: 39523388 Free PMC article.
-
Dynamic optimization elucidates higher-level pathogenicity strategies of Pseudomonas aeruginosa.Microlife. 2025 Mar 13;6:uqaf005. doi: 10.1093/femsml/uqaf005. eCollection 2025. Microlife. 2025. PMID: 40182079 Free PMC article.
-
Regulation of Heme Synthesis by Mitochondrial Homeostasis Proteins.Front Cell Dev Biol. 2022 Jun 27;10:895521. doi: 10.3389/fcell.2022.895521. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35832791 Free PMC article. Review.
References
-
- Harris JW. X-linked, pyridoxine-responsive sideroblastic anemia. N Engl J Med. 1994;330(10):709-711. - PubMed
-
- Ferreira GC, Andrew TL, Karr SW, Dailey HA.. Organization of the terminal two enzymes of the heme biosynthetic pathway. Orientation of protoporphyrinogen oxidase and evidence for a membrane complex. J Biol Chem. 1988;263(8):3835-3839. - PubMed
-
- Kramer MF, Gunaratne P, Ferreira GC.. Transcriptional regulation of the murine erythroid-specific 5-aminolevulinate synthase gene. Gene. 2000; 247(1-2):153-166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

