Cysteamine Decreases Low-Density Lipoprotein Oxidation, Causes Regression of Atherosclerosis, and Improves Liver and Muscle Function in Low-Density Lipoprotein Receptor-Deficient Mice
- PMID: 34493066
- PMCID: PMC8649511
- DOI: 10.1161/JAHA.120.017524
Cysteamine Decreases Low-Density Lipoprotein Oxidation, Causes Regression of Atherosclerosis, and Improves Liver and Muscle Function in Low-Density Lipoprotein Receptor-Deficient Mice
Abstract
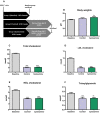
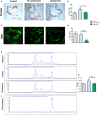
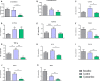
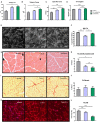
Background We have shown previously that low-density lipoprotein (LDL) can be oxidized in the lysosomes of macrophages, that this oxidation can be inhibited by cysteamine, an antioxidant that accumulates in lysosomes, and that this drug decreases atherosclerosis in LDL receptor-deficient mice fed a high-fat diet. We have now performed a regression study with cysteamine, which is of more relevance to the treatment of human disease. Methods and Results LDL receptor-deficient mice were fed a high-fat diet to induce atherosclerotic lesions. They were then reared on chow diet and drinking water containing cysteamine or plain drinking water. Aortic atherosclerosis was assessed, and samples of liver and skeletal muscle were analyzed. There was no regression of atherosclerosis in the control mice, but cysteamine caused regression of between 32% and 56% compared with the control group, depending on the site of the lesions. Cysteamine substantially increased markers of lesion stability, decreased ceroid, and greatly decreased oxidized phospholipids in the lesions. The liver lipid levels and expression of cluster of differentiation 68, acetyl-coenzyme A acetyltransferase 2, cytochromes P450 (CYP)27, and proinflammatory cytokines and chemokines were decreased by cysteamine. Skeletal muscle function and oxidative fibers were increased by cysteamine. There were no changes in the plasma total cholesterol, LDL cholesterol, high-density lipoprotein cholesterol, or triacylglycerol concentrations attributable to cysteamine. Conclusions Inhibiting the lysosomal oxidation of LDL in atherosclerotic lesions by antioxidants targeted at lysosomes causes the regression of atherosclerosis and improves liver and muscle characteristics in mice and might be a promising novel therapy for atherosclerosis in patients.
Keywords: antioxidant; atherosclerosis; lipoprotein; low‐density lipoprotein.
Conflict of interest statement
None.
Figures


References
-
- Kleemann R, Verschuren L, van Erk MJ, Nikolsky Y, Cnubben NHP, Verheij ER, Smilde AK, Hendriks HFJ, Zadelaar S, Smith GJ, et al. Atherosclerosis and liver inflammation induced by increased dietary cholesterol intake: a combined transcriptomics and metabolomics analysis. Genome Biol. 2007;8:R200. DOI: 10.1186/gb-2007-8-9-r200. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

