Circ-ABCB10 knockdown inhibits the malignant progression of cervical cancer through microRNA-128-3p/ZEB1 axis
- PMID: 34493213
- PMCID: PMC8422762
- DOI: 10.1186/s12575-021-00154-8
Circ-ABCB10 knockdown inhibits the malignant progression of cervical cancer through microRNA-128-3p/ZEB1 axis
Erratum in
-
Correction: Circ‑ABCB10 knockdown inhibits the malignant progression of cervical cancer through microRNA‑128‑3p/ZEB1 axis.Biol Proced Online. 2023 Aug 4;25(1):23. doi: 10.1186/s12575-023-00216-z. Biol Proced Online. 2023. PMID: 37542238 Free PMC article. No abstract available.
Abstract
Aims: We focused on the detailed functions of circ-ABCB10 in cervical cancer (CC) development and its mechanisms.
Background: The increasing findings have proposed the central roles of circular RNAs (circRNAs) in the tumorigenesis of various human cancers. Circ-ABCB10 displays promising oncogenic effect in several tumors.
Methods: Circ-ABCB10 and miR-128-3p production levels in CC tissues and cells were tested through RT-qPCR. The association of circ-ABCB10 expression with clinicopathologic parameters of CC patients was statistically analyzed. Cell proliferation, invasion, apoptosis, and epithelial-mesenchymal transition (EMT) were evaluated by MTT, transwell invasion assays, flow cytometry analyses, and western blot examination of EMT markers. The binding activity between miR-128-3p and circ-ABCB10 or zinc finger E-box binding homeobox 1 (ZEB1) was explored through pull-down assay or luciferase reporter assay. The influence of circ-ABCB10 on CC tumorigenesis was evaluated by in vivo xenograft experiments.
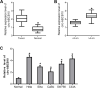
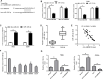
Results: The elevated circ-ABCB10 expression was determined in CC tissues and cells. Moreover, higher production level of circ-ABCB10 was close related to lymph-node metastasis, Federation of Gynecology and Obstetrics (FIGO) stage, and tumor size in CC patients. Loss of circ-ABCB10 weakened cell proliferative and invasive abilities, inhibited EMT, and induced apoptosis in CC. Loss of circ-ABCB10 inhibited ZEB1 expression by serving as a sponge of miR-128-3p in CC cells. Circ-ABCB10 sponged miR-128-3p to enhance cell proliferation, invasion, EMT and inhibit apoptosis in CC cells. Xenograft tumor assays confirmed that circ-ABCB10 knockdown inhibited CC tumor growth.
Conclusion: Our study suggests that circ-ABCB10 depletion inhibits proliferation, invasion and EMT and promotes apoptosis of cervical cancer cells through miR-128-3p/ZEB1 axis and represses CC tumor growth.
Keywords: Apoptosis; Cervical cancer; Circ-ABCB10; Invasion; Proliferation; ZEB1; miR-128-3p.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing or financial interests.
Figures




Similar articles
-
CircRNA DNA methyltransferase 1 silence inhibits breast cancer development by regulating micoRNA-485-3p/zinc finger E-box binding homeobox 1 axis.J Obstet Gynaecol Res. 2021 Mar;47(3):1068-1081. doi: 10.1111/jog.14639. Epub 2021 Jan 5. J Obstet Gynaecol Res. 2021. PMID: 33403756
-
circ-MYBL2 Serves As A Sponge For miR-361-3p Promoting Cervical Cancer Cells Proliferation And Invasion.Onco Targets Ther. 2019 Nov 20;12:9957-9964. doi: 10.2147/OTT.S218976. eCollection 2019. Onco Targets Ther. 2019. PMID: 31819492 Free PMC article.
-
Knockdown of circ-ABCB10 promotes sensitivity of lung cancer cells to cisplatin via miR-556-3p/AK4 axis.BMC Pulm Med. 2020 Jan 13;20(1):10. doi: 10.1186/s12890-019-1035-z. BMC Pulm Med. 2020. PMID: 31931771 Free PMC article.
-
Oxymatrine induces anti-tumor response in cervical cancer by modulating circ_0008460/miR-197-3p/ribonucleotide reductase subunit M2 (RRM2).Bioengineered. 2022 May;13(5):12912-12926. doi: 10.1080/21655979.2022.2078943. Bioengineered. 2022. PMID: 35609310 Free PMC article.
-
Circ_0006646 Accelerates the Growth and Metastasis of Cervical Cancer by Elevating RRM2 Through miR-758-3p.Biochem Genet. 2023 Aug;61(4):1300-1318. doi: 10.1007/s10528-022-10320-6. Epub 2022 Dec 30. Biochem Genet. 2023. PMID: 36583788
Cited by
-
Emerging role and clinical applications of circular RNAs in human diseases.Funct Integr Genomics. 2025 Mar 28;25(1):77. doi: 10.1007/s10142-025-01575-4. Funct Integr Genomics. 2025. PMID: 40148685 Review.
-
Circ_0081723 enhances cervical cancer progression and modulates CREBRF via sponging miR-545-3p.Naunyn Schmiedebergs Arch Pharmacol. 2024 Nov;397(11):8839-8852. doi: 10.1007/s00210-024-03175-8. Epub 2024 Jun 8. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38850307
-
miR-128-3p Reduces Proliferation and Immune Escape in Acute Myeloid Leukemia Through Targeted Regulation of ZEB1.Appl Biochem Biotechnol. 2025 Aug;197(8):4999-5016. doi: 10.1007/s12010-025-05255-8. Epub 2025 May 17. Appl Biochem Biotechnol. 2025. PMID: 40381097
-
Prognostic value of circWWC3 as a novel survival‑related biomarker for clear cell renal cell carcinoma.Oncol Lett. 2023 Apr 3;25(5):196. doi: 10.3892/ol.2023.13782. eCollection 2023 May. Oncol Lett. 2023. PMID: 37113397 Free PMC article.
-
Hsa_circ_0001925 promotes malignant progression in triple-negative breast cancer via miR-1299/YY1 axis.Thorac Cancer. 2023 Mar;14(8):746-757. doi: 10.1111/1759-7714.14803. Epub 2023 Feb 8. Thorac Cancer. 2023. PMID: 36754085 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

