Detection of Leishmania tarentolae in lizards, sand flies and dogs in southern Italy, where Leishmania infantum is endemic: hindrances and opportunities
- PMID: 34493323
- PMCID: PMC8423600
- DOI: 10.1186/s13071-021-04973-2
Detection of Leishmania tarentolae in lizards, sand flies and dogs in southern Italy, where Leishmania infantum is endemic: hindrances and opportunities
Abstract
Background: Leishmania tarentolae is a protozoan isolated from geckoes (Tarentola annularis, Tarentola mauritanica), which is considered non-pathogenic and is transmitted by herpetophilic Sergentomyia spp. sand flies. This species occurs in sympatry with Leishmania infantum in areas where canine leishmaniasis is endemic. In the present study, we investigated the circulation of L. tarentolae and L. infantum in sand flies, dogs and lizards in a dog shelter in southern Italy, where canine leishmaniasis by L. infantum is endemic.
Methods: Sheltered dogs (n = 100) negative for Leishmania spp. (March 2020) were screened by immunofluorescence antibody test (IFAT) using promastigotes of both species at two time points (June 2020 and March 2021). Whole blood from dogs, tissues of Podarcis siculus lizards (n = 28) and sand flies (n = 2306) were also sampled and tested by a duplex real-time PCR (dqPCR). Host blood meal was assessed in sand flies by PCR.
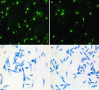
Results: Overall, 16 dogs became positive for L. infantum and/or L. tarentolae by IFAT at one or both sampling periods. One canine blood sample was positive for L. infantum, whilst two for L. tarentolae by dqPCR. At the cytology of lizard blood, Leishmania spp. amastigote-like forms were detected in erythrocytes. Twenty-two tissue samples, mostly lung (21.4%), scored molecularly positive for L. tarentolae, corresponding to 10 lizards (i.e., 35.7%). Of the female Sergentomyia minuta sampled (n = 1252), 158 scored positive for L. tarentolae, four for L. infantum, and one co-infected. Two Phlebotomus perniciosus (out of 29 females) were positive for L. tarentolae. Engorged S. minuta (n = 10) fed on humans, and one P. perniciosus, positive for L. tarentolae, on lagomorphs.
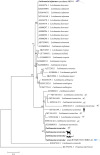
Conclusions: Dogs and lacertid lizards (Podarcis siculus) were herein found for the first time infected by L. tarentolae. The detection of both L. tarentolae and L. infantum in S. minuta and P. perniciosus suggests their sympatric circulation, with a potential overlap in vertebrate hosts. The interactions between L. tarentolae and L. infantum should be further investigated in both vectors and vertebrate hosts to understand the potential implications for the diagnosis and control of canine leishmaniasis in endemic areas.
Keywords: Canine leishmaniasis; IFAT; Leishmania infantum; Leishmania tarentolae; Reptiles; Sergentomyia minuta; Zoonosis; dqPCR.
© 2021. The Author(s).
Conflict of interest statement
The sponsor played no role in the study design, data interpretation or conclusions. Fred Beugnet is a Boehringer-Ingelheim employee. The authors declare that they have no competing interests.
Figures


Similar articles
-
Leishmania (Sauroleishmania) tarentolae isolation and sympatric occurrence with Leishmania (Leishmania) infantum in geckoes, dogs and sand flies.PLoS Negl Trop Dis. 2022 Aug 9;16(8):e0010650. doi: 10.1371/journal.pntd.0010650. eCollection 2022 Aug. PLoS Negl Trop Dis. 2022. PMID: 35943980 Free PMC article.
-
Detection and isolation of Leishmania infantum and Leishmania tarentolae in sand flies from a canine leishmaniasis endemic area.Acta Trop. 2025 Aug;268:107704. doi: 10.1016/j.actatropica.2025.107704. Epub 2025 Jun 22. Acta Trop. 2025. PMID: 40555290
-
Molecular detection of Leishmania (Sauroleishmania) tarentolae in human blood and Leishmania (Leishmania) infantum in Sergentomyia minuta: unexpected host-parasite contacts.Med Vet Entomol. 2020 Dec;34(4):470-475. doi: 10.1111/mve.12464. Epub 2020 Jul 24. Med Vet Entomol. 2020. PMID: 32710462
-
Leishmania tarentolae: A new frontier in the epidemiology and control of the leishmaniases.Transbound Emerg Dis. 2022 Sep;69(5):e1326-e1337. doi: 10.1111/tbed.14660. Epub 2022 Aug 3. Transbound Emerg Dis. 2022. PMID: 35839512 Free PMC article. Review.
-
Canine leishmaniasis in the Americas: etiology, distribution, and clinical and zoonotic importance.Parasit Vectors. 2024 Apr 30;17(1):198. doi: 10.1186/s13071-024-06282-w. Parasit Vectors. 2024. PMID: 38689318 Free PMC article. Review.
Cited by
-
Live attenuated-nonpathogenic Leishmania and DNA structures as promising vaccine platforms against leishmaniasis: innovations can make waves.Front Microbiol. 2024 Apr 3;15:1326369. doi: 10.3389/fmicb.2024.1326369. eCollection 2024. Front Microbiol. 2024. PMID: 38633699 Free PMC article. Review.
-
Diversity of CRESS DNA Viruses in Squamates Recapitulates Hosts Dietary and Environmental Sources of Exposure.Microbiol Spectr. 2022 Jun 29;10(3):e0078022. doi: 10.1128/spectrum.00780-22. Epub 2022 May 26. Microbiol Spectr. 2022. PMID: 35616383 Free PMC article.
-
Sand Fly Fauna and Prevalence of Leishmania spp. in a Newly Investigated Area of Northern Italy: Emerging Epidemiological Scenarios?Transbound Emerg Dis. 2025 Jul 21;2025:4426385. doi: 10.1155/tbed/4426385. eCollection 2025. Transbound Emerg Dis. 2025. PMID: 40727309 Free PMC article.
-
Diversity of biting midges, mosquitoes and sand flies at four dog shelters in rural and peri-urban areas of Central Morocco.Parasite. 2024;31:57. doi: 10.1051/parasite/2024057. Epub 2024 Sep 27. Parasite. 2024. PMID: 39331804 Free PMC article.
-
Leishmania Seroprevalence in Dogs: Comparing Shelter and Domestic Communities.Animals (Basel). 2023 Jul 19;13(14):2352. doi: 10.3390/ani13142352. Animals (Basel). 2023. PMID: 37508129 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

