Outcome measurement instrument selection for lung physiology in systemic sclerosis associated interstitial lung disease: A systematic review using the OMERACT filter 2.1 process
- PMID: 34493396
- PMCID: PMC8678187
- DOI: 10.1016/j.semarthrit.2021.08.001
Outcome measurement instrument selection for lung physiology in systemic sclerosis associated interstitial lung disease: A systematic review using the OMERACT filter 2.1 process
Abstract
Objective: The Outcome Measures in Rheumatology (OMERACT) is a research organization focused on improving health care outcomes for patients with autoimmune and musculoskeletal diseases. The Connective Tissue Disease-Interstitial Lung Disease (CTD-ILD) Working Group on Lung Physiology is a group within OMERACT charged with identifying outcome measures that should be implemented in studies of patients with CTD-ILD. The OMERACT Filter 2.1 is an evidence-based algorithm used to identify outcome measures that are truthful, feasible, and able to discriminate between groups of interest. Our objective was to summate evidence (published literature, key opinion leader input, patient perspectives) that would influence the CTD-ILD Working Group's vote to accept or reject the use of two measures of lung physiology, the forced vital capacity (FVC) and the diffusion capacity of carbon monoxide (DLco) for use in randomized controlled trials (RTCs) and longitudinal observational studies (LOSs) involving patients with systemic sclerosis associated ILD (SSc-ILD).
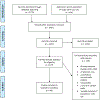
Methods: Patient Research Partners (those afflicted with SSc-ILD) and the CTD-ILD Working Group on Lung Physiology were polled to assess their opinion on the FVC and DLco in terms of feasibility; the CTD-ILD Working Group was also queried on these instruments' face and content validity. We then conducted a systematic literature review to identify articles in the SSc-ILD population that assessed the following measurement properties of FVC and DLco: (1) construct validity, (2) test-retest reliability, (3) longitudinal construct validity, (4) clinical trial discrimination/sensitivity to detect change in clinical trials, and (5) thresholds of meaning. Results were summarized in a Summary of Measurement Properties (SOMP) table for each instrument. OMERACT CTD-ILD Working Group members discussed and voted on the strength of evidence supporting these two instruments and voted to endorse, provisionally endorse, or not endorse either instrument.
Results: Forty Patient Research Partners reported these two measures are feasible (are not an unnecessary burden or represent an infeasible longitudinal assessment of their disease). A majority of the 18 CTD-ILD Working Group members voted that both the FVC and DLco are feasible and have face and content validity. The systematic literature review returned 1,447 non-duplicated articles, of which 177 met eligibility for full text review. Forty-eight studies (13 RCTs, 35 LOSs) were included in the qualitative analysis. The FVC SOMP table revealed high quality, consistent data with evidence of good performance for all five measurement properties, suggesting requisite published evidence to proceed with endorsement. The DLco SOMP table showed a lack of data to support test-retest reliability and inadequate evidence to support clinical trial discrimination. There was unanimous agreement (15 [100%]) among voting CTD-ILD Working Group members to endorse the FVC as an instrument for lung physiology in RCTs and LOSs in SSc-ILD. Based on currently available evidence, DLco did not meet the OMERACT criteria and is not recommended for use in RCTs to represent lung physiology of SSc-ILD. The OMERACT Technical Advisory Group agreed with these decisions.
Conclusion: The OMERACT Filter 2.1 was successfully applied to the domain of lung physiology in patients with SSc-ILD. The FVC was endorsed for use in RCTs and LOSs based on the Working Group's vote; DLco was not endorsed.
Keywords: Core set; Diffusion capacity of carbon monoxide; Forced vital capacity; OMERACT; Outcome measures; Systemic sclerosis interstitial lung disease.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Please see each author's ICJME form.
Figures
Similar articles
-
Predictors of lung function test severity and outcome in systemic sclerosis-associated interstitial lung disease.PLoS One. 2017 Aug 1;12(8):e0181692. doi: 10.1371/journal.pone.0181692. eCollection 2017. PLoS One. 2017. PMID: 28763468 Free PMC article.
-
Performance of Forced Vital Capacity and Lung Diffusion Cutpoints for Associated Radiographic Interstitial Lung Disease in Systemic Sclerosis.J Rheumatol. 2018 Nov;45(11):1572-1576. doi: 10.3899/jrheum.171362. Epub 2018 Oct 1. J Rheumatol. 2018. PMID: 30275265 Free PMC article.
-
Connective Tissue Disease-associated Interstitial Lung Diseases (CTD-ILD) - Report from OMERACT CTD-ILD Working Group.J Rheumatol. 2015 Nov;42(11):2168-71. doi: 10.3899/jrheum.141182. Epub 2015 Mar 1. J Rheumatol. 2015. PMID: 25729034 Free PMC article. Review.
-
PsAID12 Provisionally Endorsed at OMERACT 2018 as Core Outcome Measure to Assess Psoriatic Arthritis-specific Health-related Quality of Life in Clinical Trials.J Rheumatol. 2019 Aug;46(8):990-995. doi: 10.3899/jrheum.181077. Epub 2018 Dec 15. J Rheumatol. 2019. PMID: 30554154 Free PMC article. Review.
-
Pulmonary function tests as outcomes for systemic sclerosis interstitial lung disease.Eur Respir Rev. 2018 May 15;27(148):170102. doi: 10.1183/16000617.0102-2017. Print 2018 Jun 30. Eur Respir Rev. 2018. PMID: 29769294 Free PMC article.
Cited by
-
Assessment of disease outcome measures in systemic sclerosis.Nat Rev Rheumatol. 2022 Sep;18(9):527-541. doi: 10.1038/s41584-022-00803-6. Epub 2022 Jul 20. Nat Rev Rheumatol. 2022. PMID: 35859133 Review.
-
Risk factors for lung function decline in systemic sclerosis-associated interstitial lung disease in a large single-centre cohort.Rheumatology (Oxford). 2023 Jul 5;62(7):2501-2509. doi: 10.1093/rheumatology/keac639. Rheumatology (Oxford). 2023. PMID: 36377780 Free PMC article.
References
-
- Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390(10103):1685–1699. - PubMed
-
- Wells AU. Interstitial lung disease in systemic sclerosis. Press Medicale. 2014;43(10):e329–e343. - PubMed
-
- Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: A study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69(10):1809–1815. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous