Single session compared with multiple sessions of education and exercise for older adults with spinal pain in an advanced practice physiotherapy model of care: protocol for a randomised controlled trial
- PMID: 34493525
- PMCID: PMC8424421
- DOI: 10.1136/bmjopen-2021-053004
Single session compared with multiple sessions of education and exercise for older adults with spinal pain in an advanced practice physiotherapy model of care: protocol for a randomised controlled trial
Abstract
Objectives: To assess the effectiveness and cost-effectiveness of a single session compared with multiple sessions of education and exercise for older adults with spinal pain treated conservatively in an advanced practice physiotherapy model of care.
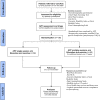
Methods and analysis: In this pragmatic randomised controlled trial, 152 older adults (≥65 years old) with neck or back pain initially referred for a consultation in neurosurgery, but treated conservatively, will be recruited through the advanced practice physiotherapy neurosurgery CareAxis programme in the Montreal region (Quebec, Canada). In the CareAxis programme, older patients with spinal pain are triaged by an advance practice physiotherapist and are offered conservative care and only potential surgical candidates are referred to a neurosurgeon. Participants will be randomised into one of two arms: 1-a single session or 2-multiple sessions (6 sessions over 12 weeks) of education and exercise with the advance practice physiotherapist (1:1 ratio). The primary outcome measure will be the Brief Pain Inventory (pain severity and interference subscales). Secondary measures will include self-reported disability (the Neck Disability Index or Oswestry Disability Index), the Pain Catastrophizing Scale, satisfaction with care questionnaires (9-item Visit-specific Satisfaction Questionnaire and MedRisk), and the EQ-5D-5L. Participants' healthcare resources use and related costs will be measured. Outcomes will be collected at baseline and at 6, 12 and 26 weeks after enrolment. Intention-to-treat analyses will be performed, and repeated mixed-model analysis of variance will assess differences between treatment arms. Cost-utility analyses will be conducted from the perspective of the healthcare system.
Ethics and dissemination: Ethics approval has been obtained from the Comité d'éthique de la recherche du CIUSS de l'Est-de-l'Île-de-Montréal (FWA00001935 and IRB00002087). Results of this study will be presented to different stakeholders, published in peer-reviewed journals and presented at international conferences.
Protocol version: V.4 August 2021.
Trial registration number: NCT04868591; Pre-results.
Keywords: health economics; musculoskeletal disorders; organisation of health services; rehabilitation medicine.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: CS declares being the Chief Medical Officer for CareAxis, a non-profit organisation involving an APP model of care for adults with spinal pain, and being a consultant for Medtronic and for Stryker, two medical devices and technology firms.
Figures
Similar articles
-
Face-to-face physiotherapy compared with a supported home exercise programme for the management of musculoskeletal conditions: protocol of a multicentre, randomised controlled trial-the REFORM trial.BMJ Open. 2021 May 18;11(5):e041242. doi: 10.1136/bmjopen-2020-041242. BMJ Open. 2021. PMID: 34006536 Free PMC article.
-
Effectiveness of a physiotherapist-led triage and treatment service on WAITing time for adults with musculoskeletal pain referred to Australian public hospital physiotherapy clinics: a protocol for the WAIT-less trial.BMJ Open. 2025 Jan 15;15(1):e091293. doi: 10.1136/bmjopen-2024-091293. BMJ Open. 2025. PMID: 39819907 Free PMC article.
-
Better Outcomes for Older people with Spinal Trouble (BOOST) Trial: a randomised controlled trial of a combined physical and psychological intervention for older adults with neurogenic claudication, a protocol.BMJ Open. 2018 Oct 18;8(10):e022205. doi: 10.1136/bmjopen-2018-022205. BMJ Open. 2018. PMID: 30341124 Free PMC article.
-
Clinical and cost-effectiveness of progressive exercise compared with best practice advice, with or without corticosteroid injection, for the treatment of rotator cuff disorders: protocol for a 2x2 factorial randomised controlled trial (the GRASP trial).BMJ Open. 2017 Jul 17;7(7):e018004. doi: 10.1136/bmjopen-2017-018004. BMJ Open. 2017. PMID: 28716796 Free PMC article. Clinical Trial.
-
Advanced practice physiotherapy for adults with spinal pain: a systematic review with meta-analysis.Eur Spine J. 2021 Apr;30(4):990-1003. doi: 10.1007/s00586-020-06648-5. Epub 2020 Oct 29. Eur Spine J. 2021. PMID: 33123757
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials