Assisted gene flow using cryopreserved sperm in critically endangered coral
- PMID: 34493583
- PMCID: PMC8463791
- DOI: 10.1073/pnas.2110559118
Assisted gene flow using cryopreserved sperm in critically endangered coral
Abstract
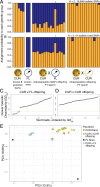
Assisted gene flow (AGF) is a conservation intervention to accelerate species adaptation to climate change by importing genetic diversity into at-risk populations. Corals exemplify both the need for AGF and its technical challenges; corals have declined in abundance, suffered pervasive reproductive failures, and struggled to adapt to climate change, yet mature corals cannot be easily moved for breeding, and coral gametes lose viability within hours. Here, we report the successful demonstration of AGF in corals using cryopreserved sperm that was frozen for 2 to 10 y. We fertilized Acropora palmata eggs from the western Caribbean (Curaçao) with cryopreserved sperm from genetically distinct populations in the eastern and central Caribbean (Florida and Puerto Rico, respectively). We then confirmed interpopulation parentage in the Curaçao-Florida offspring using 19,696 single-nucleotide polymorphism markers. Thus, we provide evidence of reproductive compatibility of a Caribbean coral across a recognized barrier to gene flow. The 6-mo survival of AGF offspring was 42%, the highest ever achieved in this species, yielding the largest wildlife population ever raised from cryopreserved material. By breeding a critically endangered coral across its range without moving adults, we show that AGF using cryopreservation is a viable conservation tool to increase genetic diversity in threatened marine populations.
Keywords: Acropora palmata; assisted gene flow; coral reproduction; cryopreservation; endangered species.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Aitken S., Whitlock M., Assisted gene flow to facilitate local adaptation to climate change. Annu. Rev. Ecol. Evol. Syst. 44, 367–388 (2013).
-
- Palumbi S. R., Barshis D. J., Traylor-Knowles N., Bay R. A., Mechanisms of reef coral resistance to future climate change. Science 344, 895–898 (2014). - PubMed
-
- Anthony K., et al., New interventions are needed to save coral reefs. Nat. Ecol. Evol. 1, 1420–1422 (2017). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

