Cannabinoid Metabolites as Inhibitors of Major Hepatic CYP450 Enzymes, with Implications for Cannabis-Drug Interactions
- PMID: 34493602
- PMCID: PMC11022895
- DOI: 10.1124/dmd.121.000442
Cannabinoid Metabolites as Inhibitors of Major Hepatic CYP450 Enzymes, with Implications for Cannabis-Drug Interactions
Abstract
The legalization of cannabis in many parts of the United States and other countries has led to a need for a more comprehensive understanding of cannabis constituents and their potential for drug-drug interactions. Although (-)-trans-Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD), and cannabinol (CBN) are the most abundant cannabinoids present in cannabis, THC metabolites are found in plasma at higher concentrations and for a longer duration than that of the parent cannabinoids. To understand the potential for drug-drug interactions, the inhibition potential of major cannabinoids and their metabolites on major hepatic cytochrome P450 (P450) enzymes was examined. In vitro assays with P450-overexpressing cell microsomes demonstrated that the major THC metabolites 11-hydroxy-Δ9-tetra-hydrocannabinol and 11-nor-9-carboxy-Δ9-THC-glucuronide competitively inhibited several major P450 enzymes, including CYP2B6, CYP2C9, and CYP2D6 (apparent Ki,u values = 0.086 ± 0.066 µM and 0.90 ± 0.54 µM, 0.057 ± 0.044 µM and 2.1 ± 0.81 µM, 0.15 ± 0.067 µM and 2.3 ± 0.54 µM, respectively). 11-Nor-9-carboxy-Δ9- tetrahydrocannabinol exhibited no inhibitory activity against any CYP450 tested. THC competitively inhibited CYP1A2, CYP2B6, CYP2C9, and CYP2D6; CBD competitively inhibited CYP3A4, CYP2B6, CYP2C9, CYP2D6, and CYP2E1; and CBN competitively inhibited CYP2B6, CYP2C9, and CYP2E1. THC and CBD showed mixed-type inhibition for CYP2C19 and CYP1A2, respectively. These data suggest that cannabinoids and major THC metabolites are able to inhibit the activities of multiple P450 enzymes, and basic static modeling of these data suggest the possibility of pharmacokinetic interactions between these cannabinoids and xenobiotics extensively metabolized by CYP2B6, CYP2C9, and CYP2D6. SIGNIFICANCE STATEMENT: Major cannabinoids and their metabolites found in the plasma of cannabis users inhibit several P450 enzymes, including CYP2B6, CYP2C9, and CYP2D6. This study is the first to show the inhibition potential of the most abundant plasma cannabinoid metabolite, THC-COO-Gluc, and suggests that circulating metabolites of cannabinoids play an essential role in CYP450 enzyme inhibition as well as drug-drug interactions.
Copyright © 2021 by The Author(s).
Figures

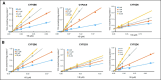
References
-
- Aizpurua-Olaizola O, Soydaner U, Öztürk E, Schibano D, Simsir Y, Navarro P, Etxebarria N, Usobiaga A (2016) Evolution of the cannabinoid and terpene content during the growth of cannabis sativa plants from different chemotypes. J Nat Prod 79:324–331. - PubMed
-
- Code EL, Crespi CL, Penman BW, Gonzalez FJ, Chang TKH, Waxman DJ (1997) Human cytochrome P4502B6: interindividual hepatic expression, substrate specificity, and role in procarcinogen activation. Drug Metab Dispos 25:985–993. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

