Monogenic Epilepsies: Disease Mechanisms, Clinical Phenotypes, and Targeted Therapies
- PMID: 34493617
- PMCID: PMC10336826
- DOI: 10.1212/WNL.0000000000012744
Monogenic Epilepsies: Disease Mechanisms, Clinical Phenotypes, and Targeted Therapies
Abstract
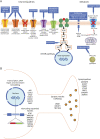
A monogenic etiology can be identified in up to 40% of people with severe epilepsy. To address earlier and more appropriate treatment strategies, clinicians are required to know the implications that specific genetic causes might have on pathophysiology, natural history, comorbidities, and treatment choices. In this narrative review, we summarize concepts on the genetic epilepsies based on the underlying pathophysiologic mechanisms and present the current knowledge on treatment options based on evidence provided by controlled trials or studies with lower classification of evidence. Overall, evidence robust enough to guide antiseizure medication (ASM) choices in genetic epilepsies remains limited to the more frequent conditions for which controlled trials and observational studies have been possible. Most monogenic disorders are very rare and ASM choices for them are still based on inferences drawn from observational studies and early, often anecdotal, experiences with precision therapies. Precision medicine remains applicable to only a narrow number of patients with monogenic epilepsies and may target only part of the actual functional defects. Phenotypic heterogeneity is remarkable, and some genetic mutations activate epileptogenesis through their developmental effects, which may not be reversed postnatally. Other genes seem to have pure functional consequences on excitability, acting through either loss- or gain-of-function effects, and these may have opposite treatment implications. In addition, the functional consequences of missense mutations may be difficult to predict, making precision treatment approaches considerably more complex than estimated by deterministic interpretations. Knowledge of genetic etiologies can influence the approach to surgical treatment of focal epilepsies. Identification of germline mutations in specific genes contraindicates surgery while mutations in other genes do not. Identification, quantification, and functional characterization of specific somatic mutations before surgery using CSF liquid biopsy or after surgery in brain specimens will likely be integrated in planning surgical strategies and reintervention after a first unsuccessful surgery as initial evidence suggests that mutational load may correlate with the epileptogenic zone. Promising future directions include gene manipulation by DNA or mRNA targeting; although most are still far from clinical use, some are in early phase clinical development.
© 2021 American Academy of Neurology.
Conflict of interest statement
R. Guerrini has acted as an investigator for studies with Zoogenic, Biocodex, UCB, Angelini, and Eisai Inc.; and has been a speaker and on advisory boards for Zoogenic, Biocodex, Novartis, Biomarin, and GW Pharma. S. Balestrini reports personal fees from Biocodex, Eisai, and UCB Pharma, outside the submitted work. E. Wirrell has served as a consultant for Biocodex and Biomarin and is a member of the Independent Adjudication Committee for the Envision study, supported by Encoded Therapeutics. M. Walker reports grants from Vitaflo, personal fees from UCB Pharma, personal fees from Eisai, personal fees from GSK, personal fees from GW Pharmaceuticals, and personal fees from Marinus Pharmaceuticals, outside the submitted work; and has patents “Composition for the use in the treatment of epilepsy” WO2018189113A1 licensed to Vitaflo, “Combination comprising decanoic acid for the treatment of epilepsy” WO2019002435A1 pending, “Therapeutic use of compounds” EP2642990B1 issued, “Combined use of a vector encoding a modified receptor and its exogenous agonist in the treatment of seizures” US10525103B2 issued, and “Expression vectors comprising engineered genes WO2018229254A1” pending. Go to
Figures
References
-
- Guo MH, Bardakjian TM, Brzozowski MR, et al. . Temporal trends and yield of clinical diagnostic genetic testing in adult neurology. Am J Med Genet. Epub 2021 Jun 2. - PubMed
-
- US National Library of Medicine. Accessed July 27, 2021. clinicaltrials.gov/
