Lipids modulate the BH3-independent membrane targeting and activation of BAX and Bcl-xL
- PMID: 34493661
- PMCID: PMC8449356
- DOI: 10.1073/pnas.2025834118
Lipids modulate the BH3-independent membrane targeting and activation of BAX and Bcl-xL
Abstract
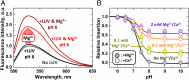
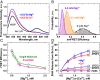
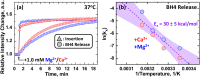
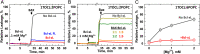
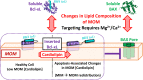
Regulation of apoptosis is tightly linked with the targeting of numerous Bcl-2 proteins to the mitochondrial outer membrane (MOM), where their activation or inhibition dictates cell death or survival. According to the traditional view of apoptotic regulation, BH3-effector proteins are indispensable for the cytosol-to-MOM targeting and activation of proapoptotic and antiapoptotic members of the Bcl-2 protein family. This view is challenged by recent studies showing that these processes can occur in cells lacking BH3 effectors by as yet to be determined mechanism(s). Here, we exploit a model membrane system that recapitulates key features of MOM to demonstrate that the proapoptotic Bcl-2 protein BAX and antiapoptotic Bcl-xL have an inherent ability to interact with membranes in the absence of BH3 effectors, but only in the presence of cellular concentrations of Mg2+/Ca2+ Under these conditions, BAX and Bcl-xL are selectively targeted to membranes, refolded, and activated in the presence of anionic lipids especially the mitochondrial-specific lipid cardiolipin. These results provide a mechanistic explanation for the mitochondrial targeting and activation of Bcl-2 proteins in cells lacking BH3 effectors. At cytosolic Mg2+ levels, the BH3-independent activation of BAX could provide localized amplification of apoptotic signaling at regions enriched in cardiolipin (e.g., contact sites between MOM and mitochondrial inner membrane). Increases in MOM cardiolipin, as well as cytosolic [Ca2+] during apoptosis could further contribute to its MOM targeting and activity. Meanwhile, the BH3-independent targeting and activation of Bcl-xL to the MOM is expected to counter the action of proapoptotic BAX, thereby preventing premature commitment to apoptosis.
Keywords: apoptosis; divalent cations; membrane protein folding; mitochondria permeabilization; protein–lipid interactions.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
The cytosolic domain of Bcl-2 forms small pores in model mitochondrial outer membrane after acidic pH-induced membrane association.Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2009 Feb;26(1):130-7. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2009. PMID: 19334571 Free PMC article.
-
After embedding in membranes antiapoptotic Bcl-XL protein binds both Bcl-2 homology region 3 and helix 1 of proapoptotic Bax protein to inhibit apoptotic mitochondrial permeabilization.J Biol Chem. 2014 Apr 25;289(17):11873-11896. doi: 10.1074/jbc.M114.552562. Epub 2014 Mar 10. J Biol Chem. 2014. PMID: 24616095 Free PMC article.
-
The multidomain proapoptotic molecules Bax and Bak are directly activated by heat.Proc Natl Acad Sci U S A. 2005 Dec 13;102(50):17975-80. doi: 10.1073/pnas.0506712102. Epub 2005 Dec 5. Proc Natl Acad Sci U S A. 2005. PMID: 16330765 Free PMC article.
-
Discoveries and controversies in BCL-2 protein-mediated apoptosis.FEBS J. 2016 Jul;283(14):2690-700. doi: 10.1111/febs.13527. Epub 2015 Oct 27. FEBS J. 2016. PMID: 26411300 Review.
-
Involvement of cardiolipin in tBID-induced activation of BAX during apoptosis.Chem Phys Lipids. 2014 Apr;179:70-4. doi: 10.1016/j.chemphyslip.2013.12.002. Epub 2013 Dec 12. Chem Phys Lipids. 2014. PMID: 24333953 Review.
Cited by
-
Ca2+ -dependent interactions between lipids and the tumor-targeting peptide pHLIP.Protein Sci. 2022 Sep;31(9):e4385. doi: 10.1002/pro.4385. Protein Sci. 2022. PMID: 36040255 Free PMC article.
-
A kinetic fluorescence polarization ligand assay for monitoring BAX early activation.Cell Rep Methods. 2022 Mar 28;2(3):100174. doi: 10.1016/j.crmeth.2022.100174. Epub 2022 Mar 9. Cell Rep Methods. 2022. PMID: 35419554 Free PMC article.
-
Therapeutic Effects of Bee Bread on Obesity-Induced Testicular-Derived Oxidative Stress, Inflammation, and Apoptosis in High-Fat Diet Obese Rat Model.Antioxidants (Basel). 2022 Jan 28;11(2):255. doi: 10.3390/antiox11020255. Antioxidants (Basel). 2022. PMID: 35204140 Free PMC article.
-
Membrane interactions of apoptotic inhibitor Bcl-xL: What can be learned using fluorescence spectroscopy.BBA Adv. 2023 Jan 13;3:100076. doi: 10.1016/j.bbadva.2023.100076. eCollection 2023. BBA Adv. 2023. PMID: 37082264 Free PMC article.
-
The C-terminal sequences of Bcl-2 family proteins mediate interactions that regulate cell death.Biochem J. 2024 Jul 17;481(14):903-922. doi: 10.1042/BCJ20210352. Biochem J. 2024. PMID: 38985308 Free PMC article. Review.
References
-
- Tsujimoto Y., Cossman J., Jaffe E., Croce C. M., Involvement of the bcl-2 gene in human follicular lymphoma. Science 228, 1440–1443 (1985). - PubMed
-
- Vaux D. L., Cory S., Adams J. M., Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 335, 440–442 (1988). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

