Clean and folded: Production of active, high quality recombinant human interferon-λ1
- PMID: 34493923
- PMCID: PMC8411590
- DOI: 10.1016/j.procbio.2021.08.029
Clean and folded: Production of active, high quality recombinant human interferon-λ1
Abstract
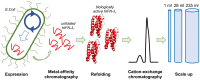
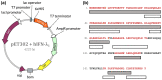
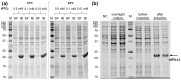
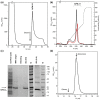
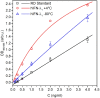
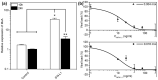
Type III interferons exhibit antiviral activity against influenza viruses, coronaviruses, rotaviruses, and others. In addition, this type of interferon theoretically has therapeutic advantages, in comparison with type I interferons, due to its ability to activate a narrower group of genes in a relatively small group of target cells. Hence, it can elicit more targeted antiviral or immunomodulatory responses. Obtaining biologically-active interferon lambda (hIFN-λ1) is fraught with difficulties at the stage of expression in soluble form or, in the case of expression in the form of inclusion bodies, at the stage of refolding. In this work, hIFN-λ1 was expressed in the form of inclusion bodies, and a simple, effective refolding method was developed. Efficient and scalable methods for chromatographic purification of recombinant hIFN-λ1 were also developed. High-yield, high-purity product was obtained through optimization of several processes including: recombinant protein expression; metal affinity chromatography; cation exchange chromatography; and an intermediate protein refolding stage. The obtained protein was shown to feature expected specific biological activity in line with published effects: induction of MxA gene expression in A549 cells and antiviral activity against influenza A virus.
Keywords: Cation exchange chromatography; Immobilized metal affinity chromatography; Interferon-lambda; Refolding.
© 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors report no declarations of interest.
Figures
References
LinkOut - more resources
Full Text Sources

