This is a preprint.
Early post-infection treatment of SARS-CoV-2 infected macaques with human convalescent plasma with high neutralizing activity reduces lung inflammation
- PMID: 34494025
- PMCID: PMC8423222
- DOI: 10.1101/2021.09.01.458520
Early post-infection treatment of SARS-CoV-2 infected macaques with human convalescent plasma with high neutralizing activity reduces lung inflammation
Update in
-
Early post-infection treatment of SARS-CoV-2 infected macaques with human convalescent plasma with high neutralizing activity had no antiviral effects but moderately reduced lung inflammation.PLoS Pathog. 2022 Apr 20;18(4):e1009925. doi: 10.1371/journal.ppat.1009925. eCollection 2022 Apr. PLoS Pathog. 2022. PMID: 35443018 Free PMC article.
Abstract
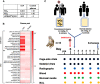
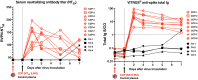
Early in the SARS-CoV-2 pandemic, there was a high level of optimism based on observational studies and small controlled trials that treating hospitalized patients with convalescent plasma from COVID-19 survivors (CCP) would be an important immunotherapy. However, as more data from controlled trials became available, the results became disappointing, with at best moderate evidence of efficacy when CCP with high titers of neutralizing antibodies was used early in infection. To better understand the potential therapeutic efficacy of CCP, and to further validate SARS-CoV-2 infection of macaques as a reliable animal model for testing such strategies, we inoculated 12 adult rhesus macaques with SARS-CoV-2 by intratracheal and intranasal routes. One day later, 8 animals were infused with pooled human CCP with a high titer of neutralizing antibodies (RVPN NT 50 value of 3,003), while 4 control animals received normal human plasma. Animals were monitored for 7 days. Animals treated with CCP had detectable levels of antiviral antibodies after infusion. In comparison to the control animals, they had similar levels of virus replication in the upper and lower respiratory tract, but had significantly reduced interstitial pneumonia, as measured by comprehensive lung histology. By highlighting strengths and weaknesses, data of this study can help to further optimize nonhuman primate models to provide proof-of-concept of intervention strategies, and guide the future use of convalescent plasma against SARS-CoV-2 and potentially other newly emerging respiratory viruses.
Author summary: The results of treating SARS-CoV-2 infected hospitalized patients with COVID-19 convalescent plasma (CCP), collected from survivors of natural infection, have been disappointing. The available data from various studies indicate at best moderate clinical benefits only when CCP with high titer of neutralizing antibodies was infused early in infection. The macaque model of SARS-CoV-2 infection can be useful to gain further insights in the value of CCP therapy. In this study, animals were infected with SARS-CoV-2 and the next day, were infused with pooled human convalescent plasma, selected to have a very high titer of neutralizing antibodies. While administration of CCP did not result in a detectable reduction in virus replication in the respiratory tract, it significantly reduced lung inflammation. These data, combined with the results of monoclonal antibody studies, emphasize the need to use products with high titers of neutralizing antibodies, and guide the future development of CCP-based therapies.
Figures




References
-
- Johns Hopkins University of Medicine. Coronavirus Resource Center [cited 2021 February 23]. Available from: https://coronavirus.jhu.edu/.
-
- Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211(1):80–90. Epub 2014/07/18. doi: 10.1093/infdis/jiu396. - DOI - PMC - PubMed
-
- FDA issues Emergency Use Authorization for Convalescent Plasma as potential promising COVID–19 Treatment, Another achievement in administration’s fight against pandemic [Internet]. August 23, 2020. Available from: https://www.fda.gov/news-events/press-announcements/fda-issues-emergency...
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
