This is a preprint.
Prediction of vaccine efficacy of the Delta variant
- PMID: 34494029
- PMCID: PMC8423227
- DOI: 10.1101/2021.08.26.21262699
Prediction of vaccine efficacy of the Delta variant
Abstract
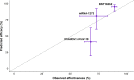
The emergence of SARS-CoV-2 variants have raised concerns over the protective efficacy of the current generation of vaccines, and it remains unclear to what extent, if any, different variants impact the efficacy and effectiveness of various SARS-CoV-2 vaccines. We systematically searched for studies of SARS-CoV-2 vaccine efficacy and effectiveness, as well as neutralization data for variants, and used a previously published statistical model to predict vaccine efficacy against variants. Overall, we estimate the efficacy of mRNA-1273 and ChAdOx1 nCoV-19 against infection caused by the Delta variant to be 25-50% lower than that of prototype strains. The predicted efficacy against symptomatic illness of the mRNA vaccines BNT162b2 and mRNA-1273 are 95.1% (UI: 88.4-98.1%) and 80.8% (60.7-92.3%), respectively, which are higher than that of adenovirus-vector vaccines Ad26.COV2.S (44.8%, UI: 29.8-60.1%) and ChAdOx1 nCoV-19 (41.1%, 19.8-62.8%). Taken together, these results suggest that the development of more effective vaccine strategies against the Delta variant may be needed. Finally, the use of neutralizing antibody titers to predict efficacy against variants provides an additional tool for public health decision making, as new variants continue to emerge.
Figures
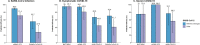
Similar articles
-
Prediction of long-term kinetics of vaccine-elicited neutralizing antibody and time-varying vaccine-specific efficacy against the SARS-CoV-2 Delta variant by clinical endpoint.BMC Med. 2022 Jan 28;20(1):36. doi: 10.1186/s12916-022-02249-9. BMC Med. 2022. PMID: 35086547 Free PMC article.
-
Comparison of BNT162b2-, mRNA-1273- and Ad26.COV2.S-Elicited IgG and Neutralizing Titers against SARS-CoV-2 and Its Variants.Vaccines (Basel). 2022 May 27;10(6):858. doi: 10.3390/vaccines10060858. Vaccines (Basel). 2022. PMID: 35746466 Free PMC article.
-
Neutralizing antibodies to SARS-CoV-2 variants of concern including Delta and Omicron in subjects receiving mRNA-1273, BNT162b2, and Ad26.COV2.S vaccines.J Med Virol. 2022 Dec;94(12):5678-5690. doi: 10.1002/jmv.28032. Epub 2022 Aug 6. J Med Virol. 2022. PMID: 35902378 Free PMC article.
-
Immunogenicity and clinical features relating to BNT162b2 messenger RNA COVID-19 vaccine, Ad26.COV2.S and ChAdOx1 adenoviral vector COVID-19 vaccines: a systematic review of non-interventional studies.Futur J Pharm Sci. 2022;8(1):20. doi: 10.1186/s43094-022-00409-5. Epub 2022 Mar 26. Futur J Pharm Sci. 2022. PMID: 35368622 Free PMC article. Review.
-
Role of COVID-19 Vaccines in SARS-CoV-2 Variants.Front Immunol. 2022 May 20;13:898192. doi: 10.3389/fimmu.2022.898192. eCollection 2022. Front Immunol. 2022. PMID: 35669787 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
