Rab7b regulates dendritic cell migration by linking lysosomes to the actomyosin cytoskeleton
- PMID: 34494097
- PMCID: PMC8487646
- DOI: 10.1242/jcs.259221
Rab7b regulates dendritic cell migration by linking lysosomes to the actomyosin cytoskeleton
Abstract
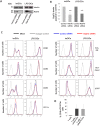
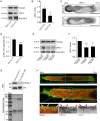
Lysosomal signaling facilitates the migration of immune cells by releasing Ca2+ to activate the actin-based motor myosin II at the cell rear. However, how the actomyosin cytoskeleton physically associates to lysosomes is unknown. We have previously identified myosin II as a direct interactor of Rab7b, a small GTPase that mediates the transport from late endosomes/lysosomes to the trans-Golgi network (TGN). Here, we show that Rab7b regulates the migration of dendritic cells (DCs) in one- and three-dimensional environments. DCs are immune sentinels that transport antigens from peripheral tissues to lymph nodes to activate T lymphocytes and initiate adaptive immune responses. We found that the lack of Rab7b reduces myosin II light chain phosphorylation and the activation of the transcription factor EB (TFEB), which controls lysosomal signaling and is required for fast DC migration. Furthermore, we demonstrate that Rab7b interacts with the lysosomal Ca2+ channel TRPML1 (also known as MCOLN1), enabling the local activation of myosin II at the cell rear. Taken together, our findings identify Rab7b as the missing physical link between lysosomes and the actomyosin cytoskeleton, allowing control of immune cell migration through lysosomal signaling. This article has an associated First Person interview with the first author of the paper.
Keywords: Actomyosin; Cell migration; Dendritic cells; Rab protein; Rab7b.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures





References
-
- Berg-Larsen, A., Landsverk, O. J. B., Progida, C., Gregers, T. F. and Bakke, O. (2013). Differential regulation of Rab GTPase expression in monocyte-derived dendritic cells upon lipopolysaccharide activation: a correlation to maturation-dependent functional properties. PLoS ONE 8, e73538. 10.1371/journal.pone.0073538 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

