Diabetes concomitant to aortic stenosis is associated with increased expression of NF-κB and more pronounced valve calcification
- PMID: 34494136
- PMCID: PMC8494674
- DOI: 10.1007/s00125-021-05545-w
Diabetes concomitant to aortic stenosis is associated with increased expression of NF-κB and more pronounced valve calcification
Abstract
Aims/hypothesis: Type 2 diabetes has been demonstrated to predispose to aortic valve calcification. We investigated whether type 2 diabetes concomitant to aortic stenosis (AS) enhances valvular inflammation and coagulation activation via upregulated expression of NF-κB, with subsequent increased expression of bone morphogenetic protein 2 (BMP-2).
Methods: In this case-control study, 50 individuals with severe isolated AS and concomitant type 2 diabetes were compared with a control group of 100 individuals without diabetes. The median (IQR) duration of diabetes since diagnosis was 11 (7-18) years, and 36 (72%) individuals had HbA1c ≥48 mmol/mol (≥6.5%). Stenotic aortic valves obtained during valve replacement surgery served for in loco NF-κB, BMP-2, prothrombin (FII) and active factor X (FXa) immunostaining. In vitro cultures of valve interstitial cells (VICs), isolated from obtained valves were used for mechanistic experiments and PCR investigations.
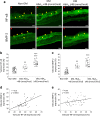
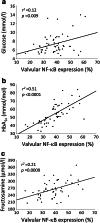
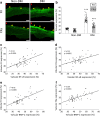
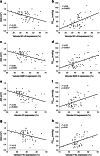
Results: Diabetic compared with non-diabetic individuals displayed enhanced valvular expression of NF-κB, BMP-2, FII and FXa (all p ≤ 0.001). Moreover, the expression of NF-κB and BMP-2 positively correlated with amounts of valvular FII and FXa. Only in diabetic participants, valvular NF-κB expression was strongly associated with serum levels of HbA1c, and moderately with fructosamine. Of importance, in diabetic participants, valvular expression of NF-κB correlated with aortic valve area (AVA) and maximal transvalvular pressure gradient. In vitro experiments conducted using VIC cultures revealed that glucose (11 mmol/l) upregulated expression of both NF-κB and BMP-2 (p < 0.001). In VIC cultures treated with glucose in combination with reactive oxygen species (ROS) inhibitor (N-acetyl-L-cysteine), the expression of NF-κB and BMP-2 was significantly suppressed. A comparable effect was observed for VICs cultured with glucose in combination with NF-κB inhibitor (BAY 11-7082), suggesting that high doses of glucose activate oxidative stress leading to proinflammatory actions in VICs. Analysis of mRNA expression in VICs confirmed these findings; glucose caused a 6.9-fold increase in expression of RELA (NF-κB p65 subunit), with the ROS and NF-κB inhibitor reducing the raised expression of RELA by 1.8- and 3.2-fold, respectively.
Conclusions/interpretation: Type 2 diabetes enhances in loco inflammation and coagulation activation within stenotic valve leaflets. Increased valvular expression of NF-κB in diabetic individuals is associated not only with serum HbA1c and fructosamine levels but also with AVA and transvalvular gradient, indicating that strict long-term glycaemic control is needed in AS patients with concomitant type 2 diabetes. This study suggests that maintaining these variables within the normal range may slow the rate of AS progression.
Keywords: Aortic stenosis; Bone morphogenetic protein 2; Coagulation factors; Diabetes mellitus; Inflammation; NF-κB; Oxidative stress.
© 2021. The Author(s).
Figures


References
-
- Baumgartner H, Hung J, Bermejo J, et al. Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis: A Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30(4):372–392. doi: 10.1093/ehjci/jew335. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

