Healthcare costs related to adverse events in hepatocellular carcinoma treatment: A retrospective observational claims study
- PMID: 34494389
- PMCID: PMC9124510
- DOI: 10.1002/cnr2.1504
Healthcare costs related to adverse events in hepatocellular carcinoma treatment: A retrospective observational claims study
Abstract
Background: Hepatocellular carcinoma (HCC) is an aggressive form of liver cancer with increasing incidence and mortality worldwide. For metastatic disease, systemic treatment is recommended. In addition to tumor characteristics, adverse events (AEs) may influence regimen choice.
Aim: To analyze healthcare burden among patients with advanced HCC, by treatment type and AEs observed.
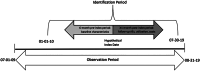
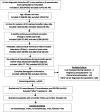
Methods: Included were adult commercial and Medicare Advantage enrollees with ≥2 non-diagnostic claims coded for HCC (the first setting the index date); ≥1 claim for systemic treatment of advanced/metastatic HCC; and continuous enrollment for a 6-month pre-index baseline period to ≥1 month post-index (follow-up). Patients were excluded by lack of systemic treatment; incomplete demographic information; pregnancy, liver transplant, other cancers during baseline or clinical trial participation. We describe patient characteristics, common AEs, overall survival, and healthcare burden in 2017 USD up to 12 months after initiation of tyrosine kinase inhibitor (TKI) monotherapy; immune checkpoint inhibitor (ICI) monotherapy; or FOLFOX combination therapy.
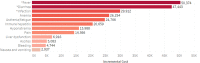
Results: The analytic sample consisted of 322 patients (median age 65.8 years, 76% male) who had 12 months' (unless death occurred prior) available follow-up, with median follow-up of 9 months. Among these, 241 (75%) had TKI monotherapy, 23 (7%) had ICI monotherapy, and 58 had FOLFOX (18%) first-line treatment. Overall, patients had a high burden of AEs (mean 3.2), with the most prevalent being pain (75%), infection (39%), ascites (34%), and bleeding (29%). After adjusting for covariates, infection ($50 374), fever ($47 443), and diarrhea ($29 912) imposed the highest incremental annual costs versus patients without the AE. Up to 90% of costs were attributable to inpatient admissions, with 56% to 60% involving intensive care. Median 1-year survival was 32%.
Conclusions: This real-world study demonstrated AE burden in alignment with previous clinical studies. Regardless of regimen used, AEs are associated with substantial healthcare costs due to inpatient care.
Keywords: adverse events; hepatocellular carcinoma; liver-directed chemotherapy.
© 2021 The Authors. Cancer Reports published by Wiley Periodicals LLC.
Conflict of interest statement
LSL, LBL, and SP were employed by Health Economics and Outcomes Research, Optum, Eden Prairie, MN; AA and BS were employed by US Medical Affairs, AstraZeneca, Gaithersburg, MD; and AT was affiliated with Hematology Oncology Associates, Carlsbad, CA, at the time this work was conducted. AA holds stock in AstraZeneca. No employment was contingent upon publication of this work. Authors declare there are no other conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

