Expanding the Chemical Space of Tetracyanobuta-1,3-diene (TCBD) through a Cyano-Diels-Alder Reaction: Synthesis, Structure, and Physicochemical Properties of an Anthryl-fused-TCBD Derivative
- PMID: 34494672
- PMCID: PMC9292653
- DOI: 10.1002/chem.202103079
Expanding the Chemical Space of Tetracyanobuta-1,3-diene (TCBD) through a Cyano-Diels-Alder Reaction: Synthesis, Structure, and Physicochemical Properties of an Anthryl-fused-TCBD Derivative
Abstract
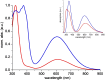
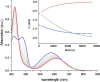
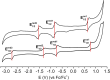
Tetracyanobuta-1,3-diene (TCBD) is a powerful and versatile electron-acceptor moiety widely used for the preparation of electroactive conjugates. While many reports addressing its electron-accepting capability have appeared in the literature, significantly scarcer are those dealing with its chemical modification, a relevant topic which allows to broaden the chemical space of this interesting functional unit. Here, we report on the first example of a high-yielding cyano-Diels-Alder (CDA) reaction between TCBD, that is, where a nitrile group acts as a dienophile, and an anthryl moiety, that is, acting as a diene. The resulting anthryl-fused-TCBD derivative, which structure was unambiguously identified by X-ray diffraction, shows high thermal stability, remarkable electron-accepting capability, and interesting electronic ground- and excited-state features, as characterized by a thorough theoretical, electrochemical, and photophysical investigation. Moreover, a detailed kinetic analysis of the intramolecular CDA reaction transforming the anthryl-TCBD-based reactant into the anthryl-fused-TCBD product was carried out at different temperatures.
Keywords: anthryl-fused derivative; cyano-Diels-Alder reaction; electron acceptor; photophysics; tetracyanobuta-1,3-diene.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

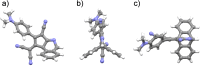


Similar articles
-
Subphthalocyanines Axially Substituted with a Tetracyanobuta-1,3-diene-Aniline Moiety: Synthesis, Structure, and Physicochemical Properties.J Am Chem Soc. 2017 Apr 19;139(15):5520-5529. doi: 10.1021/jacs.7b01460. Epub 2017 Apr 6. J Am Chem Soc. 2017. PMID: 28322560
-
Subphthalocyanine-tetracyanobuta-1,3-diene-aniline conjugates: stereoisomerism and photophysical properties.Chem Sci. 2019 Sep 19;10(48):10997-11005. doi: 10.1039/c9sc03970h. eCollection 2019 Dec 28. Chem Sci. 2019. PMID: 32055388 Free PMC article.
-
Intense Ground-State Charge-Transfer Interactions in Low-Bandgap, Panchromatic Phthalocyanine-Tetracyanobuta-1,3-diene Conjugates.Angew Chem Int Ed Engl. 2016 Apr 25;55(18):5560-4. doi: 10.1002/anie.201601258. Epub 2016 Mar 24. Angew Chem Int Ed Engl. 2016. PMID: 27010677
-
Application of the aza-Diels-Alder reaction in the synthesis of natural products.Org Biomol Chem. 2017 Apr 11;15(15):3105-3129. doi: 10.1039/c6ob02761j. Org Biomol Chem. 2017. PMID: 28327756 Review.
-
Progress in Lewis-Acid-Templated Diels-Alder Reactions.Molecules. 2024 Mar 6;29(5):1187. doi: 10.3390/molecules29051187. Molecules. 2024. PMID: 38474699 Free PMC article. Review.
Cited by
-
Synthesis and Optical Characterization of Hydrazone-Substituted Push-Pull-Type NLOphores.J Org Chem. 2024 Sep 20;89(18):13192-13207. doi: 10.1021/acs.joc.4c01328. Epub 2024 Sep 10. J Org Chem. 2024. PMID: 39255504 Free PMC article.
-
Perspectives on push-pull chromophores derived from click-type [2 + 2] cycloaddition-retroelectrocyclization reactions of electron-rich alkynes and electron-deficient alkenes.Beilstein J Org Chem. 2024 Jan 22;20:125-154. doi: 10.3762/bjoc.20.13. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 38292046 Free PMC article. Review.
-
Unprecedented "off-pathway" [2+2] Cycloaddition-Retroelectrocyclization Reaction Between an Unsymmetric Alkyne and Tetracyanoquinodimethane.Angew Chem Int Ed Engl. 2025 Jun 10;64(24):e202506536. doi: 10.1002/anie.202506536. Epub 2025 Apr 17. Angew Chem Int Ed Engl. 2025. PMID: 40198496 Free PMC article.
-
Minimalist Design for Solar Energy Conversion: Revamping the π-Grid of an Organic Framework into Open-Shell Superabsorbers.JACS Au. 2023 Jun 5;3(6):1711-1722. doi: 10.1021/jacsau.3c00132. eCollection 2023 Jun 26. JACS Au. 2023. PMID: 37388679 Free PMC article.
References
-
- Cadranel A., Haines P., Kaur R., Menon A., Münich P. W., Schol P. R., Guldi D. M., Adv. Energy Mater. 2021, 11, 2002831.
-
- Bottari G., de la Torre G., Guldi D. M., Torres T., Coord. Chem. Rev. 2021, 428, 213605.
-
- Zieleniewska A., Lodermeyer F., Roth A., Guldi D. M., Chem. Soc. Rev. 2018, 47, 702–714. - PubMed
-
- Michinobu T., Diederich F., Angew. Chem. Int. Ed. 2018, 57, 3552–3577; - PubMed
- Angew. Chem. 2018, 130, 3612–3638.
-
- Michinobu T., Boudon C., Gisselbrecht J.-P., Seiler P., Frank B., Moonen N. N. P., Gross M., Diederich F., Chem. Eur. J. 2006, 12, 1889–1905. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

