Analysis of the Role of N-Linked Glycosylation in Cell Surface Expression, Function, and Binding Properties of SARS-CoV-2 Receptor ACE2
- PMID: 34494876
- PMCID: PMC8557876
- DOI: 10.1128/Spectrum.01199-21
Analysis of the Role of N-Linked Glycosylation in Cell Surface Expression, Function, and Binding Properties of SARS-CoV-2 Receptor ACE2
Abstract
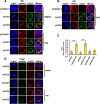
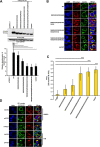
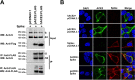
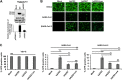
Human angiotensin I-converting enzyme 2 (hACE2) is a type I transmembrane glycoprotein that serves as the major cell entry receptor for SARS-CoV and SARS-CoV-2. The viral spike (S) protein is required for the attachment to ACE2 and subsequent virus-host cell membrane fusion. Previous work has demonstrated the presence of N-linked glycans in ACE2. N-glycosylation is implicated in many biological activities, including protein folding, protein activity, and cell surface expression of biomolecules. However, the contribution of N-glycosylation to ACE2 function is poorly understood. Here, we examined the role of N-glycosylation in the activity and localization of two species with different susceptibility to SARS-CoV-2 infection, porcine ACE2 (pACE2) and hACE2. The elimination of N-glycosylation by tunicamycin (TM) treatment, or mutagenesis, showed that N-glycosylation is critical for the proper cell surface expression of ACE2 but not for its carboxiprotease activity. Furthermore, nonglycosylable ACE2 was localized predominantly in the endoplasmic reticulum (ER) and not at the cell surface. Our data also revealed that binding of SARS-CoV or SARS-CoV-2 S protein to porcine or human ACE2 was not affected by deglycosylation of ACE2 or S proteins, suggesting that N-glycosylation does not play a role in the interaction between SARS coronaviruses and the ACE2 receptor. Impairment of hACE2 N-glycosylation decreased cell-to-cell fusion mediated by SARS-CoV S protein but not that mediated by SARS-CoV-2 S protein. Finally, we found that hACE2 N-glycosylation is required for an efficient viral entry of SARS-CoV/SARS-CoV-2 S pseudotyped viruses, which may be the result of low cell surface expression of the deglycosylated ACE2 receptor. IMPORTANCE Understanding the role of glycosylation in the virus-receptor interaction is important for developing approaches that disrupt infection. In this study, we showed that deglycosylation of both ACE2 and S had a minimal effect on the spike-ACE2 interaction. In addition, we found that the removal of N-glycans of ACE2 impaired its ability to support an efficient transduction of SARS-CoV and SARS-CoV-2 S pseudotyped viruses. Our data suggest that the role of deglycosylation of ACE2 on reducing infection is likely due to a reduced expression of the viral receptor on the cell surface. These findings offer insight into the glycan structure and function of ACE2 and potentially suggest that future antiviral therapies against coronaviruses and other coronavirus-related illnesses involving inhibition of ACE2 recruitment to the cell membrane could be developed.
Keywords: ACE2 glycoprotein; ACE2-spike binding; N-glycosylation; coronavirus; syncytium formation; virus entry.
Figures






References
-
- World Health Organization. 2020. World Health Organization coronavirus disease 2019 (COVID-19) situation report. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio....
-
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team. 2020. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733. doi: 10.1056/NEJMoa2001017. - DOI - PMC - PubMed
-
- Wu F, Zhao S, Yu B, Chen Y-M, Wang W, Song Z-G, Hu Y, Tao Z-W, Tian J-H, Pei Y-Y, Yuan M-L, Zhang Y-L, Dai F-H, Liu Y, Wang Q-M, Zheng J-J, Xu L, Holmes EC, Zhang Y-Z. 2020. A new coronavirus associated with human respiratory disease in China. Nature 579:265–269. doi: 10.1038/s41586-020-2008-3. - DOI - PMC - PubMed
-
- Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, Chen H-D, Chen J, Luo Y, Guo H, Jiang R-D, Liu M-Q, Chen Y, Shen X-R, Wang X, Zheng X-S, Zhao K, Chen Q-J, Deng F, Liu L-L, Yan B, Zhan F-X, Wang Y-Y, Xiao G-F, Shi Z-L. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. doi: 10.1038/s41586-020-2012-7. - DOI - PMC - PubMed
-
- Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. 2020. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 130:2620–2629. doi: 10.1172/JCI137244. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

