Disinfection of Maternal Environments Is Associated with Piglet Microbiome Composition from Birth to Weaning
- PMID: 34494881
- PMCID: PMC8550216
- DOI: 10.1128/mSphere.00663-21
Disinfection of Maternal Environments Is Associated with Piglet Microbiome Composition from Birth to Weaning
Abstract
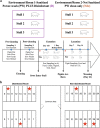
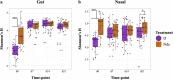
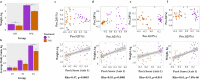
Maternal factors predetermine offspring development and health, including the establishment of offsprings' first microbiomes. Research in swine has shown that early microbial exposures impact microbiome colonization in piglets, but this phenomenon has never been tested in the context of delivery room disinfection. Thus, we exposed gestating sows to two delivery environments (n = 3/environment): stalls cleaned with a broad-spectrum disinfectant (disinfected environment [D]) or stalls cleaned only with hot-water power washing (nondisinfected environment [Nde]), 3 days prior to farrowing. Microbiomes of sows and farrowed piglets (n = 27/environment) were profiled at 4 different time points from birth to weaning via 16S rRNA sequencing. The results show that although vaginal, milk, skin, and gut microbiomes in mothers were minimally affected, sanitation of farrowing stalls impacted piglet microbiome colonization. These effects were mainly characterized by lower bacterial diversity in the gut and nasal cavity, specifically in D piglets at birth, and by distinct taxonomic compositions from birth to weaning depending on the farrowing environment. For instance, environmental bacteria greatly influenced microbiome colonization in Nde piglets, which also harbored significantly higher abundances of gut Lactobacillus and nasal Enhydrobacter at several time points through weaning. Different sanitation strategies at birth also resulted in distinct microbiome assembly patterns, with lower microbial exposures in D piglets being associated with limited interactions between bacterial taxa. However, increasing microbial exposures at birth through the lack of disinfection were also associated with lower piglet weight, highlighting the importance of understanding the trade-offs among optimal microbiome development, health, and growth performance in swine production systems. IMPORTANCE We show that levels of disinfection in farrowing facilities can impact early microbial exposures and colonization by pioneer microbes in piglets. Although previous research has shown a similar effect by raising pigs outdoors or by exposing them to soil, these practices are unattainable in most swine production systems in the United States due to biosecurity practices. Thus, our results underscore the importance of evaluating different disinfection practices in swine production to safely reduce pathogenic risks without limiting early microbial exposures. Allowing early exposure to both beneficial and pathogenic microbes may positively impact immune responses, reduce the stressors of weaning, and potentially reduce the need for dietary antimicrobials. However, the benefits of modified early microbial exposures need to be accomplished along with acceptable growth performance. Thus, our results also provide clues for understanding how disinfection practices in farrowing rooms may impact early microbiome development and assembly.
Keywords: disinfection; environment; gut; maternal; microbiome; piglet; programming; swine.
Figures



Similar articles
-
Lactation-related dynamics of bacterial and fungal microbiomes in feces of sows and gut colonization in suckling and newly weaned piglets.J Anim Sci. 2024 Jan 3;102:skae321. doi: 10.1093/jas/skae321. J Anim Sci. 2024. PMID: 39460650 Free PMC article.
-
An insight into the commercial piglet's microbial gut colonization: from birth towards weaning.Anim Microbiome. 2022 Dec 26;4(1):68. doi: 10.1186/s42523-022-00221-9. Anim Microbiome. 2022. PMID: 36572944 Free PMC article.
-
Early exposure to agricultural soil accelerates the maturation of the early-life pig gut microbiota.Anaerobe. 2017 Jun;45:31-39. doi: 10.1016/j.anaerobe.2017.02.022. Epub 2017 Feb 27. Anaerobe. 2017. PMID: 28249795
-
Impact of housing environment and management on pre-/post-weaning piglet productivity.J Anim Sci. 2022 Jun 1;100(6):skac142. doi: 10.1093/jas/skac142. J Anim Sci. 2022. PMID: 35708591 Free PMC article. Review.
-
Piglet gut microbial shifts early in life: causes and effects.J Anim Sci Biotechnol. 2019 Jan 14;10:1. doi: 10.1186/s40104-018-0308-3. eCollection 2019. J Anim Sci Biotechnol. 2019. PMID: 30651985 Free PMC article. Review.
Cited by
-
Selective Maternal Seeding and Rearing Environment From Birth to Weaning Shape the Developing Piglet Gut Microbiome.Front Microbiol. 2022 Apr 25;13:795101. doi: 10.3389/fmicb.2022.795101. eCollection 2022. Front Microbiol. 2022. PMID: 35547153 Free PMC article.
-
Streptococcus suis infection on European farms is associated with an altered tonsil microbiome and resistome.Microb Genom. 2024 Dec;10(12):001334. doi: 10.1099/mgen.0.001334. Microb Genom. 2024. PMID: 39699589 Free PMC article.
-
Effect of pre-farrowing hygiene routine (sub-standard vs. optimal) and creep feeding regime (dry pelleted starter diet vs. liquid mixture of milk replacer and starter diet) on post-weaning intestinal parameters and growth to slaughter in pigs.J Anim Sci. 2025 Jan 4;103:skae380. doi: 10.1093/jas/skae380. J Anim Sci. 2025. PMID: 39693382 Free PMC article.
-
Optimising Nutrition for Sustainable Pig Production: Strategies to Quantify and Mitigate Environmental Impact.Animals (Basel). 2025 May 13;15(10):1403. doi: 10.3390/ani15101403. Animals (Basel). 2025. PMID: 40427280 Free PMC article. Review.
-
Establishment of porcine fecal-derived ex vivo microbial communities to evaluate the impact of livestock feed on gut microbiome.Biosci Microbiota Food Health. 2024;43(2):100-109. doi: 10.12938/bmfh.2023-085. Epub 2023 Nov 15. Biosci Microbiota Food Health. 2024. PMID: 38577893 Free PMC article.
References
-
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. 2010. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA 107:11971–11975. doi:10.1073/pnas.1002601107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
