Effect of Online 1-Day Cognitive Behavioral Therapy-Based Workshops Plus Usual Care vs Usual Care Alone for Postpartum Depression: A Randomized Clinical Trial
- PMID: 34495285
- PMCID: PMC8427485
- DOI: 10.1001/jamapsychiatry.2021.2488
Effect of Online 1-Day Cognitive Behavioral Therapy-Based Workshops Plus Usual Care vs Usual Care Alone for Postpartum Depression: A Randomized Clinical Trial
Erratum in
-
Data Errors in Results.JAMA Psychiatry. 2021 Nov 1;78(11):1285. doi: 10.1001/jamapsychiatry.2021.2859. JAMA Psychiatry. 2021. PMID: 34586343 Free PMC article. No abstract available.
Abstract
Importance: Postpartum depression (PPD) affects as many as 20% of mothers, yet just 1 in 10 of these women receives evidence-based treatment. The COVID-19 pandemic has increased PPD risk, reduced treatment access, and shifted preferences toward virtual care.
Objective: To determine whether an online 1-day cognitive behavioral therapy (CBT)-based workshop added to treatment as usual improves PPD, anxiety, social support, mother-infant relationship quality, and infant temperament more than treatment as usual alone.
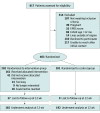
Design, setting, and participants: This randomized clinical trial included 403 women with PPD who were recruited across Ontario, Canada, during the COVID-19 pandemic (April 20 to October 4, 2020). Women with Edinburgh Postnatal Depression Scale (EPDS) scores of at least 10 who were 18 years or older and had an infant younger than 12 months were eligible.
Interventions: Women were randomly assigned to receive a live, interactive online 1-day CBT-based workshop delivered by a registered psychotherapist, psychiatrist, or clinical psychology graduate student in addition to treatment as usual (n = 202) or to receive treatment as usual and wait-listed to receive the workshop 12 weeks later (n = 201).
Main outcomes and measures: The primary outcome was change in PPD (EPDS scores) in experimental and wait list control groups 12 weeks after baseline. Secondary outcomes included maternal anxiety (7-item Generalized Anxiety Disorder Questionnaire [GAD-7]), social support (Social Provisions Scale), quality of the mother-infant relationship (Postpartum Bonding Questionnaire), and infant temperament (Infant Behavior Questionnaire-Revised Very Short Form).
Results: Participants all identified as women with a mean (SD) age of 31.8 (4.4) years. The workshop led to significant mean (SD) reductions in EPDS scores (from 16.47 [4.41] to 11.65 [4.83]; B = -4.82; P < .001) and was associated with a higher odds of exhibiting a clinically significant decrease in EPDS scores (odds ratio, 4.15; 95% CI, 2.66-6.46). The mean (SD) GAD-7 scores decreased from 12.41 (5.12) to 7.97 (5.54) after the workshop (B = -4.44; 95% CI, -5.47 to -3.38; P < .001) and participants were more likely to experience a clinically significant change (odds ratio, 3.09; 95% CI, 1.99-4.81). Mothers also reported improvements in bonding (B = -3.22; 95% CI, -4.72 to -1.71; P < .001), infant-focused anxiety (B = -1.64; 95% CI, -2.25 to 1.00; P < .001), social support (B = 3.31; 95% CI, 1.04 to 5.57; P < .001), and positive affectivity/surgency in infants (B = 0.31; 95% CI, 0.05 to 0.56; P < .001).
Conclusions and relevance: In this randomized clinical trial, an online 1-day CBT-based workshop for PPD provides an effective, brief option for mothers, reducing PPD and anxiety as well as improving social support, the mother-infant relationship, and positive affectivity/surgency in offspring.
Trial registration: ClinicalTrials.gov Identifier: NCT04485000.
Conflict of interest statement
Figures
Comment in
-
Some Concerns About Imputation Methods for Missing Data-Reply.JAMA Psychiatry. 2022 Mar 1;79(3):270-271. doi: 10.1001/jamapsychiatry.2021.3897. JAMA Psychiatry. 2022. PMID: 35019944 No abstract available.
-
Some Concerns About Imputation Methods for Missing Data.JAMA Psychiatry. 2022 Mar 1;79(3):270. doi: 10.1001/jamapsychiatry.2021.3894. JAMA Psychiatry. 2022. PMID: 35019949 No abstract available.