Autoimmunity affecting the biliary tract fuels the immunosurveillance of cholangiocarcinoma
- PMID: 34495298
- PMCID: PMC8429038
- DOI: 10.1084/jem.20200853
Autoimmunity affecting the biliary tract fuels the immunosurveillance of cholangiocarcinoma
Abstract
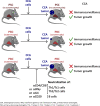
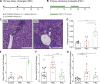
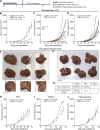
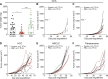
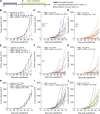
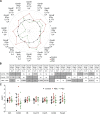
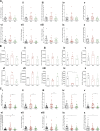
Cholangiocarcinoma (CCA) results from the malignant transformation of cholangiocytes. Primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC) are chronic diseases in which cholangiocytes are primarily damaged. Although PSC is an inflammatory condition predisposing to CCA, CCA is almost never found in the autoimmune context of PBC. Here, we hypothesized that PBC might favor CCA immunosurveillance. In preclinical murine models of cholangitis challenged with syngeneic CCA, PBC (but not PSC) reduced the frequency of CCA development and delayed tumor growth kinetics. This PBC-related effect appeared specific to CCA as it was not observed against other cancers, including hepatocellular carcinoma. The protective effect of PBC was relying on type 1 and type 2 T cell responses and, to a lesser extent, on B cells. Single-cell TCR/RNA sequencing revealed the existence of TCR clonotypes shared between the liver and CCA tumor of a PBC host. Altogether, these results evidence a mechanistic overlapping between autoimmunity and cancer immunosurveillance in the biliary tract.
© 2021 Paillet et al.
Conflict of interest statement
Disclosures: J. Tosello Boari reported personal fees from Egle Therapeutics and personal fees from Institut Curie outside the submitted work. L. Zitvogel reported "other" from everImmune, grants from Transgene, grants from Daichi Sankyo, and grants from Kaleido outside the submitted work. E. Piaggio reported personal fees from Egle Therapeutics outside the submitted work. G. Kroemer reported grants from Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Sotio, Vasculox/Tioma, Samsara Therapeutics, and Sanofi outside the submitted work; in addition, G. Kroemer is on the board of directors of the Bristol Myers Squibb Foundation France and is a scientific co-founder of everImmune, Samsara Therapeutics, and Therafast Bio. No other disclosures were reported.
Figures


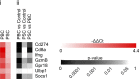


References
-
- Bae, H.R., Leung P.S., Tsuneyama K., Valencia J.C., Hodge D.L., Kim S., Back T., Karwan M., Merchant A.S., Baba N., et al. . 2016. Chronic expression of interferon-gamma leads to murine autoimmune cholangitis with a female predominance. Hepatology. 64:1189–1201. 10.1002/hep.28641 - DOI - PMC - PubMed
-
- Banales, J.M., Marin J.J.G., Lamarca A., Rodrigues P.M., Khan S.A., Roberts L.R., Cardinale V., Carpino G., Andersen J.B., Braconi C., et al. . 2020. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 17:557–588. 10.1038/s41575-020-0310-z - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

