Association of COVID-19 Lockdown With the Tumor Burden in Patients With Newly Diagnosed Metastatic Colorectal Cancer
- PMID: 34495337
- PMCID: PMC8427376
- DOI: 10.1001/jamanetworkopen.2021.24483
Association of COVID-19 Lockdown With the Tumor Burden in Patients With Newly Diagnosed Metastatic Colorectal Cancer
Abstract
Importance: The COVID-19 pandemic has been associated with substantial reduction in screening, case identification, and hospital referrals among patients with cancer. However, no study has quantitatively examined the implications of this correlation for cancer patient management.
Objective: To evaluate the association of the COVID-19 pandemic lockdown with the tumor burden of patients who were diagnosed with metastatic colorectal cancer (mCRC) before vs after lockdown.
Design, setting, and participants: This cohort study analyzed participants in the screening procedure of the PANIRINOX (Phase II Randomized Study Comparing FOLFIRINOX + Panitumumab vs FOLFOX + Panitumumab in Metastatic Colorectal Cancer Patients Stratified by RAS Status from Circulating DNA Analysis) phase 2 randomized clinical trial. These newly diagnosed patients received care at 1 of 18 different clinical centers in France and were recruited before or after the lockdown was enacted in France in the spring of 2020. Patients underwent a blood-sampling screening procedure to identify their RAS and BRAF tumor status.
Exposures: mCRC.
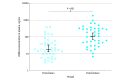
Main outcomes and measures: Circulating tumor DNA (ctDNA) analysis was used to identify RAS and BRAF status. Tumor burden was evaluated by the total plasma ctDNA concentration. The median ctDNA concentration was compared in patients who underwent screening before (November 11, 2019, to March 9, 2020) vs after (May 14 to September 3, 2020) lockdown and in patients who were included from the start of the PANIRINOX study.
Results: A total of 80 patients were included, of whom 40 underwent screening before and 40 others underwent screening after the first COVID-19 lockdown in France. These patients included 48 men (60.0%) and 32 women (40.0%) and had a median (range) age of 62 (37-77) years. The median ctDNA concentration was statistically higher in patients who were newly diagnosed after lockdown compared with those who were diagnosed before lockdown (119.2 ng/mL vs 17.3 ng/mL; P < .001). Patients with mCRC and high ctDNA concentration had lower median survival compared with those with lower concentration (14.7 [95% CI, 8.8-18.0] months vs 20.0 [95% CI, 14.1-32.0] months). This finding points to the potential adverse consequences of the COVID-19 pandemic and related lockdown.
Conclusions and relevance: This cohort study found that tumor burden differed between patients who received an mCRC diagnosis before vs after the first COVID-19 lockdown in France. The findings of this study suggest that CRC is a major area for intervention to minimize pandemic-associated delays in screening, diagnosis, and treatment.
Conflict of interest statement
Figures


References
-
- Uzzo RG, Kutinov A, Geynisman DM. COVID-19: cancer screening, diagnosis, post-treatment surveillance in uninfected patients during the pandemic and issues related to COVID-19 vaccination in cancer patients. Accessed October 9, 2020. https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-canc...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

