Comparison of Treatment Retention of Adults With Opioid Addiction Managed With Extended-Release Buprenorphine vs Daily Sublingual Buprenorphine-Naloxone at Time of Release From Jail
- PMID: 34495340
- PMCID: PMC8427378
- DOI: 10.1001/jamanetworkopen.2021.23032
Comparison of Treatment Retention of Adults With Opioid Addiction Managed With Extended-Release Buprenorphine vs Daily Sublingual Buprenorphine-Naloxone at Time of Release From Jail
Abstract
Importance: Extended-release buprenorphine (XRB), a monthly injectable long-acting opioid use disorder (OUD) treatment, has not been studied for use in corrections facilities.
Objective: To compare treatment retention following release from jail among adults receiving daily sublingual buprenorphine-naloxone (SLB) vs those receiving XRB.
Design, setting, and participants: This open-label, randomized comparative effectiveness study included 52 incarcerated adults in New York City observed for 8 weeks postrelease between June 2019 and May 2020. Participants were soon-to-be-released volunteers from 1 men's and 1 women's jail facility who had OUDs already treated with SLB. Follow-up treatment was received at a primary care clinic in Manhattan. Data were analyzed between June 2020 and December 2020.
Interventions: XRB treatment was offered prior to release and continued monthly through 8 weeks after release. SLB participants continued to receive daily directly observed in-jail SLB administration, were provided a 7-day SLB supply at jail release, and followed up at a designated clinic (or other preferred clinics).
Main outcomes and measures: Buprenorphine treatment retention at 8 weeks postrelease.
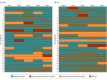
Results: A total of 52 participants were randomized 1:1 to XRB (26 participants) and SLB (26 participants). Participants had a mean (SD) age of 42.6 (10.0) years; 45 participants (87%) were men; and 40 (77%) primarily used heroin prior to incarceration. Most participants (30 [58%]) reported prior buprenorphine use; 18 (35%) reported active community buprenorphine treatment prior to jail admission. Twenty-one of 26 assigned to XRB received 1 or more XRB injection prior to release; 3 initiated XRB postrelease; and 2 did not receive XRB. Patients in the XRB arm had fewer jail medical visits compared with daily SLB medication administration (mean [SD] visits per day: XRB, 0.11 [0.03] vs SLB, 1.06 [0.08]). Community buprenorphine treatment retention at week 8 postrelease was 18 participants in the XRB group (69.2%) vs 9 in the SLB group (34.6%), and rates of opioid-negative urine tests were 72 of 130 tests in the XRB group (55.3%) and 50 of 130 tests in the SLB group (38.4%). There were no differences in rates of serious adverse events, no overdoses, and no deaths.
Conclusions and relevance: XRB was acceptable among patients currently receiving SLB, and patients had fewer in-jail clinic visits and increased community buprenorphine treatment retention when compared with standard daily SLB treatment. These results support wider use and further study of XRB as correctional and reentry OUD treatment.
Trial registration: ClinicalTrials.gov Identifier: NCT03604159.
Conflict of interest statement
Figures
Comment in
-
Opioid Use Disorder Treatment for People Involved in the US Criminal Justice System-Promising Advances and Critical Implementation Challenges.JAMA Netw Open. 2021 Sep 1;4(9):e2125120. doi: 10.1001/jamanetworkopen.2021.25120. JAMA Netw Open. 2021. PMID: 34495343 No abstract available.
References
-
- National Center for Disease Statistics. Vital Statistics Rapid Release—Provisional Drug Overdose Death Counts. Centers for Disease Control and Prevention . Last updated July 14, 2021. Accessed May 27, 2021. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm