Optimal CRT Implantation-Where and How To Place the Left-Ventricular Lead?
- PMID: 34495452
- PMCID: PMC8484220
- DOI: 10.1007/s11897-021-00528-9
Optimal CRT Implantation-Where and How To Place the Left-Ventricular Lead?
Abstract
Purpose of review: Cardiac resynchronization therapy (CRT) represents a well-established and effective non-pharmaceutical heart failure (HF) treatment in selected patients. Still, a significant number of patients remain CRT non-responders. An optimal placement of the left ventricular (LV) lead appears crucial for the intended hemodynamic and hence clinical improvement. A well-localized target area and tools that help to achieve successful lead implantation seem to be of utmost importance to reach an optimal CRT effect.
Recent findings: Recent studies suggest previous multimodal imaging (CT/cMRI/ECG torso) to guide intraprocedural LV lead placement. Relevant benefit compared to empirical lead optimization is still a matter of debate. Technical improvements in leads and algorithms (e.g., multipoint pacing (MPP), adaptive algorithms) promise higher procedural success. Recently emerging alternatives for ventricular synchronization such as conduction system pacing (CSP), LV endocardial pacing, or leadless pacing challenge classical biventricular pacing. This article reviews current strategies for a successful planning, implementation, and validation of the optimal CRT implantation. Pre-implant imaging modalities offer promising assistance for complex cases; empirical lead positioning and intraoperative testing remain the cornerstone in most cases and ensure a successful CRT effect.
Keywords: Acute hemodynamic response; Cardiac resynchronization therapy; Coronary sinus; Lead implantation; Multimodal imaging.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



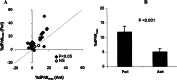
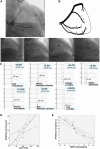
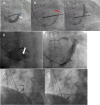

References
-
- Mower M. Method and apparatus for treating hemodynamic dysfunction by simultaneous pacing of both ventricles. US Patent #4,928,688, 29 May 1990.
-
- Writing C, Maddox TM, Januzzi JL, Jr, Allen LA, Breathett K, Butler J, et al. 2021 Update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77(6):772–810. doi: 10.1016/j.jacc.2020.11.022. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

