Aspergillus Test Profiles and Mortality in Critically Ill COVID-19 Patients
- PMID: 34495710
- PMCID: PMC8601217
- DOI: 10.1128/JCM.01229-21
Aspergillus Test Profiles and Mortality in Critically Ill COVID-19 Patients
Abstract
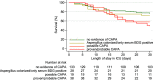
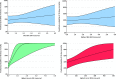
The literature regarding COVID-19-associated pulmonary aspergillosis (CAPA) has shown conflicting observations, including survival of CAPA patients not receiving antifungal therapy and discrepancy between CAPA diagnosis and autopsy findings. To gain insight into the pathophysiology of CAPA, we performed a case-control study in which we compared Aspergillus test profiles in CAPA patients and controls in relation to intensive care unit (ICU) mortality. This was a multinational case-control study in which Aspergillus test results, use of antifungal therapy, and mortality were collected from critically ill COVID-19 patients. Patients were classified using the 2020 European Confederation for Medical Mycology and the International Society for Human and Animal Mycology (ECMM/ISHAM) consensus case definitions. We analyzed 219 critically ill COVID-19 cases, including 1 proven, 38 probable, 19 possible CAPA cases, 21 Aspergillus-colonized patients, 7 patients only positive for serum (1,3)-β-d-glucan (BDG), and 133 cases with no evidence of CAPA. Mortality was 53.8% in CAPA patients compared to 24.1% in patients without CAPA (P = 0.001). Positive serum galactomannan (GM) and BDG were associated with increased mortality compared to serum biomarker-negative CAPA patients (87.5% versus 41.7%, P = 0.046; 90.0% versus 42.1%, P = 0.029, respectively). For each point increase in GM or 10-point BDG serum concentration, the odds of death increased (GM, odds ratio [OR] 10.208, 95% confidence interval [CI], 1.621 to 64.291, P = 0.013; BDG, OR, 1.247, 95% CI, 1.029 to 1.511, P = 0.024). CAPA is a complex disease, probably involving a continuum of respiratory colonization, tissue invasion, and angioinvasion. Serum biomarkers are useful for staging CAPA disease progression and, if positive, indicate angioinvasion and a high probability of mortality. There is need for a biomarker that distinguishes between respiratory tract colonization and tissue-invasive CAPA disease.
Keywords: COVID-19; critically ill; invasive pulmonary aspergillosis; mortality; mycology.
Conflict of interest statement
R.J.M.B. reports grants and other from Pfizer, MSD, and Gilead, and other from Mundipharma, Astellas, F2G, Amplyx, and Cidara outside the submitted work. A.A. reports personal fees from Gilead and Pfizer and nonfinancial support from Astellas outside the submitted work. K.L. reports personal fees from SMB Laboratoires, Gilead, FUJIFILM Wako, and Thermo Fisher Scientific and nonfinancial support from Pfizer outside the submitted work. J.B.B. reports grants from Gilead Sciences, F2G Ltd, and Thermo Fisher Scientific outside the submitted work. J.W. reports research grants and speakers’ fees from Pfizer, MSD, and Gilead outside the submitted work. P.L.W. reports personal fees from Gilead, Pfizer, F2G, IMMY, and MSD and other from Bruker, Launch, and Associates of Cape Cod outside the submitted work. F.L.V.D.V. reports personal fees from Gilead and Sobi and grants from VIDI outside the submitted work. P.E.V. reports research grants from Gilead Sciences, Astellas, MSD, F2G, and Bio-Rad; he is a speaker for Gilead Sciences and MSD and is on the advisory boards for Pfizer, MundiPharma, Cidara, MSD, and F2G. M.E., S.D., A.V.A., R.G.B., T.R., S.V.D.S.-V.D.B., N.A.F.J., K.V.D., W.J.G.M., M.H.E.R., J.A.S., A.C., and S.S. declare no conflicting interests.
Figures
References
-
- van de Veerdonk FL, Kolwijck E, Lestrade PP, Hodiamont CJ, Rijnders BJ, van Paassen J, Haas PJ, Oliveira Dos Santos C, Kampinga GA, Bergmans DC, van Dijk K, de Haan AF, van Dissel J, van der Hoeven HG, Verweij PE, Dutch Mycoses Study Group . 2017. Influenza-associated aspergillosis in critically ill patients. Am J Respir Crit Care Med 196:524–527. 10.1164/rccm.201612-2540LE. - DOI - PubMed
-
- Schauwvlieghe A, Rijnders BJA, Philips N, Verwijs R, Vanderbeke L, Van Tienen C, Lagrou K, Verweij PE, Van de Veerdonk FL, Gommers D, Spronk P, Bergmans D, Hoedemaekers A, Andrinopoulou ER, van den Berg C, Juffermans NP, Hodiamont CJ, Vonk AG, Depuydt P, Boelens J, Wauters J, Dutch-Belgian Mycosis Study Group . 2018. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med 6:782–792. 10.1016/S2213-2600(18)30274-1. - DOI - PubMed
-
- Bartoletti M, Pascale R, Cricca M, Rinaldi M, Maccaro A, Bussini L, Fornaro G, Tonetti T, Pizzilli G, Francalanci E, Giuntoli L, Rubin A, Moroni A, Ambretti S, Trapani F, Vatamanu O, Ranieri VM, Castelli A, Baiocchi M, Lewis R, Giannella M, Viale P, PREDICO study group . 2020. Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: a prospective study. Clin Infect Dis 10.1093/cid/ciaa1065. - DOI - PMC - PubMed
-
- White PL, Dhillon R, Cordey A, Hughes H, Faggian F, Soni S, Pandey M, Whitaker H, May A, Morgan M, Wise MP, Healy B, Blyth I, Price JS, Vale L, Posso R, Kronda J, Blackwood A, Rafferty H, Moffitt A, Tsitsopoulou A, Gaur S, Holmes T, Backx M. 2020. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin Infect Dis 10.1093/cid/ciaa1298. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

